Effects of Dietary Fat Types on Growth Performance, Pork Quality, and Gene Expression in Growing-finishing Pigs
Article information
Abstract
This study was performed to determine the effects of dietary fat sources, i.e., beef tallow, soybean oil, olive oil and coconut oil (each 3% in feed), on the growth performance, meat quality and gene expression in growing-finishing pigs. A total of 72 crossbred pigs (Landrace×Large White×Duroc) were used at 71±1 kg body weight (about 130 d of age) in 24 pens (320×150 cm) in a confined pig house (three pigs per pen) with six replicate pens per treatment. The growing diet was given for periods of 14±3 d and the finishing diet was given for periods of 28±3 d. The fat type had no significant effect either on growth performance or on chemical composition or on meat quality in growing-finishing pigs. Dietary fat type affected fatty acid composition, with higher levels of unsaturated fatty acids (UFAs) and monounsaturated fatty acids (MUFAs) in the olive oil group. Microarray analysis in the Longissimus dorsi identified 6 genes, related to insulin signaling pathway, that were differentially expressed among the different feed groups. Real time-PCR was conducted on the six genes in the longissimus dorsi muscle (LM). In particular, the genes encoding the protein kinase, cAMP-dependent, regulatory, type II, alpha (PRKAR2A) and the catalytic subunit of protein phosphatase 1, beta isoform (PPP1CB) showed the highest expression level in the olive oil group (respectively, p<0.05, p<0.001). The results of this study indicate that the type of dietary fat affects fatty acid composition and insulin signaling-related gene expression in the LM of pigs.
INTRODUCTION
Feeding strategy is the most commonly used management factor for quality control in the production of meat and for improvement or control of performance, animal welfare, safety, nutritional value, and eating and technological quality (Andersen et al., 2005). Fat supplementation is important for growing-finishing pigs because of the high energy value of the diet. Fats are characterized by low heat production (Noblet et al., 1994) and have the advantage of lowering the dietary heat increment of growing-finishing pigs during periods of environmental heat stress (Spencer et al., 2005).
There has been a recent increase in consumer preference for products with higher levels of unsaturated fatty acids (UFAs) because of their beneficial effects in the diet, such as prevention of cardiovascular disease (Van Deckel et al., 1996). Increased intake of polyunsaturated fatty acids (PUFAs) lowers the risk for cardiovascular disease, and there is therefore a great deal of consumer interest in these compounds (Corino et al., 2002). Pig diets supplemented with vegetable oils such as soybean, sunflower and rapeseed oils contain high levels of UFAs and may lead to healthier products for consumers. For example, soybean oil has been used as a fat source with a relatively high concentration of PUFAs (Eder et al., 2005). Although UFAs from vegetable oil affect pork quality, the effects have yet to be characterized in detail.
Meat quality of pigs is affected by breed, nutritional status, and the feeding system used in fattening. Intramuscular fat (IMF) deposition and back fat thickness (BF) are important factors in meat quality and are among the most important candidate traits for understanding the interactions between nutrition and gene expression during meat quality formation in pigs (Yin and Li, 2009). Collected data also indicate that nutritional factors can interact with other regulatory networks such as tissue-specific, developmental, and hormonal factors, as well as dietary fat or carbohydrate to regulate gene expression.
This study was performed to evaluate the impacts of dietary fat type on the growth performance, meat quality, and differential gene expression in growing-finishing pigs.
MATERIAL AND METHODS
Animals and diets
Seventy-two crossbred pigs (Landrace×Large White×Duroc) were used. The animals, at 71±1 kg body weight (about 130 d of age), were randomly allocated into 24 pens (320×150 cm with solid concrete flooring) in a confined pig house, with three pigs per pen and six replicate pens per treatment. Treatment groups consisted of the same numbers of gilts and barrows. Each pen was equipped with a nipple waterer and a stainless steel feeder, and the pigs were given free access to feed and water throughout. Animals received care in accordance with the Guide for the Care and Use of Laboratory Animals (National Institute of Animal Science Animal Care Committee).
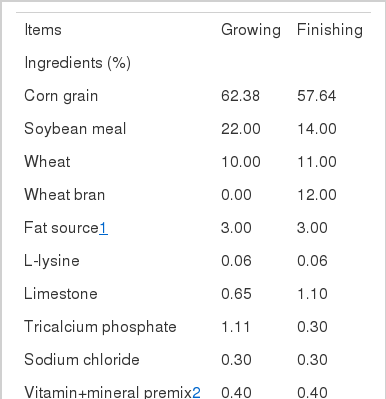
The ingredients and chemical compositions of the growing and finishing diets used in this experiment are shown in Table 1. All other nutrient requirements met or exceeded the NRC recommendations for growing and finishing pigs (NRC, 1998). Fat sources used in the present study were beef tallow, coconut oil, olive oil, and soybean oil, which were added to a concentration of 3.0% in feed. The fat sources were melted at approximately 50°C, and the melted fats were diluted approximately 10% in the diets. The 10% fat diets were then formulated to 3.0% fats supplied to each diet. The growing diet was given to crossbred pigs for experimental periods of 14±3 d. The finishing diet was given to crossbred pigs for experimental periods of 28±3 d. Pig weights and feed intakes were determined weekly to calculate the average daily gain (ADG, kg/d) and average daily feed intake (ADFI, kg/d). The ratio of gain to feed was calculated from ADG and ADFI.
Slaughtering and sampling
Pigs of 102±3 kg live weight were transported to a standard abattoir near the experimental station. The pigs were slaughtered 12 h after feed restriction. They were stunned electrically (300 V for 3 s) with a pair of stunning tongs, shackled by the right leg, and exsanguinated while hanging. The carcasses were placed in a dehairer at 62°C for 5 min, and the remaining hair was removed using a knife and flame after exit from the dehairer. The carcasses were eviscerated and split before being placed in a chiller set at 4°C for 12 h. For determination of chemical compositions and meat quality parameters, the longissimus dorsi muscle (LM, 6th to 13th rib) was removed and kept at 4°C for transport to the laboratory.
Chemical compositions and meat quality parameters
About 24 h after slaughter, the contents of moisture, crude protein, crude fat, and crude ash in samples of LM were determined according to AOAC method (AOAC, 1990). For the determination of shear force, samples were cooked individually in plastic bags immersed in a water bath at 75°C for 30 min. The cooked meat was cooled and sampled at room temperature using a 12.7-mm circular core to determine shear force. Four sample cores from each sample were sheared across the length of the core with a Warner-Bratzler shear attachment (V-type blade set) on a texture analyzer (TA-XT2i; Stable Micro Systems, Godalming, Surrey, UK), at a cross-head speed of 2 mm/s. Texture Expert for Windows™ was used to analyze the data. The shear force value, measured in units of kg, was the mean of the maximum forces required to shear each set of core samples. Cooking losses were determined as described by (Honikel, 1998). For measurement of pH24, 2-g samples of LM were homogenized at about 24 h postmortem in 10 volumes of distilled water using a Polytron homogenizer (MSE Scientific Instruments, Manor Royal, Crawley, Surrey, UK). The pH was measured using a Hanna HI 9025 pH meter (Hanna Instruments, Woonsocket, RI, USA) with an Orion 8163 glass electrode (Orion, Beverly, MA, USA). Water holding capacity (WHC) was determined by a centrifugal method as described by (Jauregui et al., 1981).
For determination of fatty acids in LM, extracted fat samples were prepared from LM after estimation of meat quality parameters. Meat fat was extracted from the ground muscle using a modification of the Folch wash method (Folch et al., 1957), as described by Ways and Hanahan (1964). Fatty acids were quantified as their fatty acid methyl esters (FAMEs), prepared by acid-catalyzed methanolysis. The FAMEs in the hexane layer were analyzed by chromatography (Agilent-6890+; Agilent Technologies, Santa Clara, CA, USA) with a flame ionization detector and split (50:1) injector. The samples were methylated in duplicate and were injected twice onto a gas-liquid chromatography (GLC) column. FAME separation was performed on an Omegawax 320 capillary GLC column (30 m×0.32 mm i.d, 0.25 mm film thickness; Supelco, Bellefonte, PA, USA) using N as the carrier gas. Flame ionization detector and injector temperatures were fixed at 260°C and 250°C, respectively. The oven temperature was set to 200°C for 40 min. Data were recorded and analyzed on a ChemStation system (G1701CA Version C.00; Agilent).
Collection of animal tissues and ribonucleic acid extraction
Immediately after slaughter, 24 LM samples were taken from animals in the four dietary groups, frozen in liquid nitrogen, and stored at −80°C until preparation of total RNA. The muscle was powdered with liquid nitrogen, and total RNA was extracted from 1 mg of muscle tissue using 3 ml of TRIzol® reagent (Invitrogen, Inc., Carlsbad, CA, USA). RNA quality was confirmed by examining the 28S and 18S rRNA bands on 1.5% agarose gels stained with ethidium bromide. Total RNA was extracted from all samples using an RNeasy Lipid Tissue Midi Kit (Qiagen, Valencia, CA, USA).
DNA microarray hybridization and microarray scanning and image analysis
Differentially expressed gene (DEG) analysis using a GeneChip® porcine genome array (Affymetrix Inc., Santa Clara, CA, USA) was performed in LM tissue from 24 pigs fed beef tallow, coconut oil, olive oil, or soybean. Aliquots of 1 μg of mRNA were converted into double-stranded cDNA using a GeneChip® Expression 3′-Amplification One Cycle Synthesis kit (Affymetrix Inc.). Hybridization cocktail (200 μl) containing 15 μg of fragmented cRNA were injected into the porcine genome array chip, and the array was scanned with a GeneChip® Scanner 3000 (Affymetrix Inc.). The fluidic station and scanner were managed by GeneChip® operating software (GCOS 1.3; Affymetrix Inc.).
Data preprocessing, clustering and biological data mining
The GeneChip® porcine genome array contains 23,937 probe sets to interrogate 23,256 transcripts in pigs, which represents 20,201 genes. Fold change and Welch’s t-test were applied to select the genes for further analysis. The genes showing 2-fold differential expression were clustered using hierarchical clustering with Pearson’s correlation as a similarity measure and complete linkage as a linkage method. GenPlex™ v2.4 software (Istech Inc., Goyang City, South Korea) was used for cluster analysis. Gene ontology significance analysis was performed to investigate the functional relationships of the 2-fold DEGs, using high-throughput GoMiner. The 2-fold DEGs were mapped to relevant pathways using GenPlex™ v2.4 software. The pathway resources were taken from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
Quantitative real time polymerase chain reaction (RT-PCR) analysis
To validate the six DEGs related to insulin signaling, based on the KEGG database, in the LM of 3 barrows in each treatment group, we performed quantitative real-time PCR (RT-PCR) with an ABI 7500 Real-Time PCR apparatus (Applied Biosystems, Foster City, CA) in 96-well plates. All primer sets were designed using sequence information from the NetAffx™ Analysis Center (http://www.affymetrix.com/analysis/index.affx) using the Primerquest program (http://www.idtdna.com/Scitools/Applications/Primerquest/). The β-actin gene (GenBank Acc. No. AY550069) was used as an internal control. Complementary DNA (cDNA) synthesis was performed by reverse transcription using SuperScript™ II reverse transcriptase (Invitrogen, Inc., Carlsbad, CA, USA) as follows. Aliquots of 4 μl of total RNA were preincubated with 0.5 μg (1 μl) of random primer mix (Promega Co., Madison, WI, USA) and 2.5 mM (1 μl) dNTP mix at 65°C for 5 min. The tubes were placed on ice, and 4 μl of 5× first-stand buffer (250 mM Tris-HCl, pH 8.3, 375 mM KCl, 15 mM MgCl2), 2 μl of 0.1 M DTT, and 40 units (0.5 μl) of RNase inhibitor (Promega) were added, followed by incubation at 42°C for 2 min. Then, 200 units (1 μl) of SuperScript™ II reverse transcriptase (Invitrogen) were added and incubation was continued at 42°C for 50 min. Reverse transcriptase activity was terminated by incubation at 70°C for 15 min (Table 6). Using this cDNA, RT-PCR was performed in a total volume of 20 μl containing 2 μl of cDNA (0.1 μg/μl), 10 μl of 2× SYBR® Green PCR Master Mix (Applied Biosystems), and 1 μl of 10 pM forward and reverse primers. The amplification reaction was started by incubation for 2 min at 50°C, followed by 40 cycles of 95°C for 10 min, 95°C for 10 s, and 60°C for 1 min. After 36 cycles, a final extension step was performed at 72°C for 1 min. RT-PCR for each gene was repeated three times. Following amplification, melting curve analysis was performed to verify the specificity of the reactions. The endpoint used in real-time RT-PCR quantification (Ct) was defined as the PCR threshold cycle number. The ΔCt value was determined by subtracting the β-actin Ct value for each sample from the target Ct value. Finally, we transformed the expression level to the 2−ΔCt value for further analysis.
Statistical analysis
Statistical analyses were performed using the general linear model (GLM) procedure with the Statistical Analysis Systems Institute software package (SAS). The data of growth performance and meat quality were subjected to analysis of least square means in a completely randomized design. The model included the effects of fat sources. The results are given as means and pooled standard errors. Differences were considered statistically significant at p<0.05.
To identify DEGs among the different dietary fat groups, statistical analysis was performed by analysis of variance (ANOVA) using the MIXED procedure with the R statistical package (http://www.R-project.org) for animals nested within age as the random effect. We also examined the least square means (LSM) for testing the significance of differences among the groups, using Duncan’s multiple range test. The following statistical model was used to estimate the effects of dietary group on individual gene expression:
Where Yij is the target gene intensity (2−ΔCt), μ is the overall mean, FEDi is the fixed effect of the ith dietary type, and DAYj is animals nested within age as a random effect.
RESULTS AND DISCUSSION
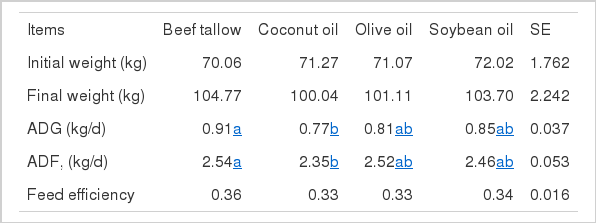
The effect of fat source on growth performance was shown in Table 2. Average daily gain (ADG, kg/d) and average daily feed intake (ADFI, kg/d) were significantly higher (p<0.05) in the beef tallow group than in the coconut oil group. However, ADG and ADFI in the soybean oil and olive oil groups were not significantly different from those in the beef tallow and coconut oil groups. Therefore, feed efficiency was not shown significant difference by type of fat source. These results consist that ADG and feed efficiency was not influenced by difference of fat sources in the pigs (Ewan, 1970; McDonald and Hamilton, 1976; Seerley et al., 1978). Also, Mitchaothai et al. (2007) reported that ADG and ADFI were not significantly different between pigs fed either a beef tallow or sunflower oil diet. When pigs were fed coconut oil in combination with tallow or corn oil, the growth rates were intermediate between those in pigs fed each fat source alone. None of the dietary lipid combinations resulted in a beneficial growth response to supplemental coconut oil, particularly when compared with long chain fatty acid of vegetable and animal lipid sources (Cera et al., 1989).
In many other reports, the addition of fat at the diet was known to improve feed efficiency during the post-weaning period (Cera et al., 1988; 1990; Howard et al., 1990; Li et al., 1990; Overland et al., 1993; Tokach et al., 1995). However, initial stage (from 0 to 14 d after weaning) was also no difference of feed efficiency. It was only improved post-weaning stage (from 14 to 35 d after weaning). This result was suggested effects by differences of energy metabolism following a growth phase.
The effects of fat source on the chemical composition of the LM are shown in Table 3. The contents of moisture, crude fat, and crude ash in the LM were similar between all treatment groups. The crude protein level in the LM was significantly higher (p<0.05) in the beef tallow group than in the soybean oil group. However, the LM crude protein levels in the olive oil and coconut oil groups were not significantly different from those in the beef tallow and soybean oil groups. Also, the values of the meat quality parameters of the LM by type of fat source are presented in Table 3. The shear force of the LM was not significantly different among the treatment groups. Cooking loss was significantly higher in the olive oil group than in the soybean oil and coconut oil groups but the effects of beef tallow on cooking loss from the LM were not significantly different from those of soybean, olive, and coconut oils. The pH24 and water holding capacity in the LM were similar between all treatment groups. It was shown that the fat type had no significant effect on chemical composition and on meat quality in the LM. Teye et al. (2006) evaluated the effects of three dietary oils (palm kernel, palm, and soybean oils) and two protein levels (high and low) on growth performance and meat quality in 60 pigs. The study which did not significantly affect growth or carcass quality by oil type is consistent with our result (Scheeder et al., 2000; De la Llata et al., 2001; Mitchaothai et al., 2007).
The fatty acid levels in the LM are presented in Table 4. The fatty acid composition of muscle and fat tissues in the pig was known to be greatly modified by incorporating the appropriate oil source in feed, since the pig is a single-stomached animal and dietary fatty acids are absorbed intact in the small intestine and then incorporated into tissue lipids (Wood et al., 1999). The myristic acid level and palmitoleic acid level were significantly higher (p<0.05 for each) in the coconut oil group than in the other three groups. Also, the palmitic acid, one of SFA, was significantly higher (p<0.05) in the beef tallow and coconut oil groups compared with the olive oil group. Coconut oil diet suggested higher deposition of SFA, especially myristic acid, and lower deposition of UFA (both MUFA and PUFA). This result agrees with the findings of Jørgensen et al. (2000) in which a coconut oil diet highest SFA, especially 14:0 and 16:0, and lowest both MUFA and PUFA in pigs. The oleic acid level was significantly higher (p<0.05) in the olive oil group than in the beef tallow and soybean oil groups, but the oleic acid level in the coconut oil group was not significantly different from those in the beef tallow, soybean oil and olive oil groups. The linoleic acid and linolenic acid levels were significantly higher (p<0.05) in the beef tallow and soybean oil groups than in the olive oil and coconut oil groups and also consistent with PUFA levels. Additionally, the stearic acid, gondoic acid, and arachidonic acid level in the LM were similar among all treatment groups. The increased linolenic acid content of pork from feeding provides the pork industry with an opportunity to provide value-added by healthful meat products for human consumption (Sun et al., 2004). So, soybean oil was suggested a great alternative of beef tallow because linolenic acid was accumulated more than the olive oil and coconut oil groups in the LM.
Olive oil represented significantly the lowest SFA level and highest level of UFA among four oil group. The use of olive oil containing a high concentration of MUFA as a fat source is related to reduced rates for cardiovascular disease and breast cancer in humans (Kiritsakis, 1999). Diets rich in olive oil have been shown to produce reduced PUFA levels in red blood cell membranes (Seiquer et al., 1996) and decrease the prevalence of coronary heart disease in the Mediterranean region (Dougherty et al., 1987). Animal fat sources such as beef tallow have low apparent digestibility, which can be improved by mixing animal fat with various vegetable oils to increase the UFA and SFA ratio via increases in lipase activity (Powles et al., 1993; Powles et al., 1994; Mountzouris et al., 1999). This result indicates that LM content could be manipulated by dietary nutrients.
We measured and compared differential gene expression in the LM in three barrows for each dietary fat type. Microarray analysis classified according to the KEGG was shown significantly expressed genes in Table 5. It revealed significantly different expression levels at 6 genes relative to insulin signaling pathway (p<0.05).
The insulin signaling pathway in pigs involves a number of genes that control glucose storage and uptake, protein synthesis, and regulation of lipid synthesis. Insulin inhibits lipid metabolism by activating a cAMP-specific phosphodiesterase in adipocytes, thereby decreasing cellular cAMP concentrations (Kitamura et al., 1999). Because insulin signaling pathway was known that closely related to fat metabolism (Wong and Sul, 2010), the six genes were performed with RT-PCR confirming that the dietary fat source influenced the expression levels of these genes in pigs. The six genes were phosphoinositide-3-kinase, catalytic, gamma polypeptide (PIK3CG); protein kinase, cAMP-dependent, regulatory, type II, alpha (PRKAR2A); AMP-activated protein kinase alpha 2 (AMPKA2); peroxisome proliferator activated receptor, gamma, coactivator 1 (PPARGC1); protein phosphatase 1, catalytic subunit, beta isoform (PPP1CB); and hormone-sensitive lipase (HSL).
The expression levels of the six DEGs were estimated using the ΔCt method, and the results are shown in Table 7. In particular, the PRKAR2A and PPP1CB genes showed marked differential expression according to dietary oil composition. It showed significant differences in expression among the four dietary oil groups, with the highest expression level in the olive oil group (Figure 1). PRKAR2A is an important signaling molecule in the regulation of metabolism and growth of mammalian cells (Hemmings et al., 1986). PPP1CB is one of the three catalytic subunits of protein phosphatase 1 (PP1), which is involved in the regulation of a variety of cellular processes such as cell division, glycogen metabolism, muscle contractility, and protein synthesis (Cohen, 1997). Especially, the PPP1CB gene is known to be related to glycogenesis, which is activated by insulin in response to high glucose levels.
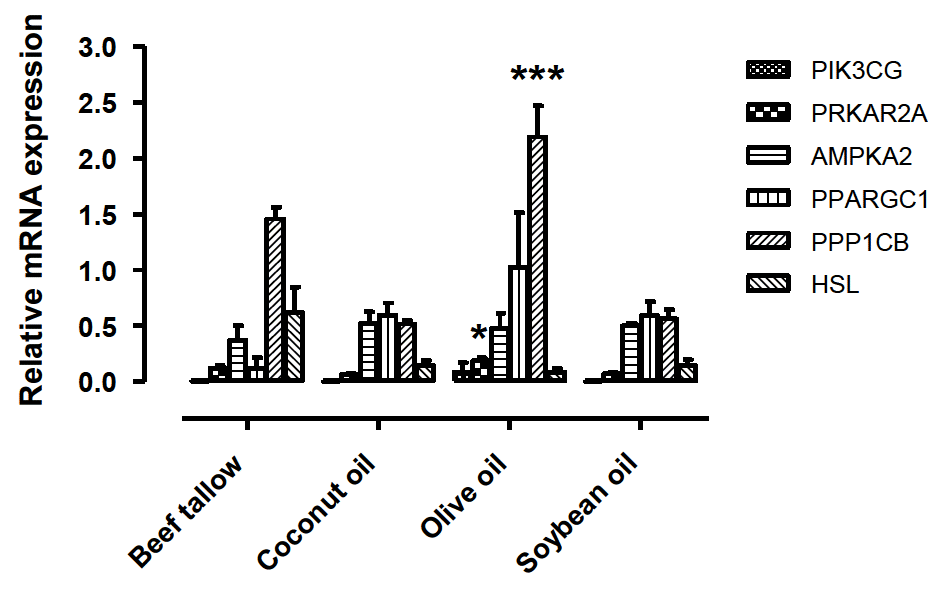
Expression patterns of differentially expressed genes with qRT-PCR at the 4 dietary oil groups. Fat sources were beef tallow, coconut oil, olive oil, and soybean oil, which were added to a concentration of 3.0% in feed. The experiments were performed using the LM 3 barrows, and data are expressed as mean±SE. The asterisks show statistically significant values (* p<0.05, ** p<0.01).
As the PUFA (linoleic and α-linolenic acid) cannot be synthesized in situ, the tissue concentrations respond rapidly to dietary changes. However, SFA and MUFA are synthesized de novo, and therefore their concentrations are less readily influenced by diet (Wood, 1984).
The PRKAR2A and PPP1CB genes showed the highest level of expression in the olive oil group with the highest levels of MUFA and the lowest level of expression in the coconut oil group with the lowest levels of MUFA. The expression of PRKAR2A and PPP1CB genes in Figure 1 is highly matched with ratio of the amount of MUFA at the oils of four types (Beef tallow 44 g/100 g, Coconut 6.6 g/100 g, Olive 69.7 g/100 g, Soybean 23.2 g/100 g) (Food Standards Agency, 1991). The oils were shown a large difference in the amount of MUFA, but the ratio of the MUFA content in LM did not shown a large difference each other in dietary group. So, these results were shown that expression of PRKAR2A and PPP1CB genes inversely matched with accumulation of MUFA in LM. Gene expression increased by absorbed a lot of MUFA, and it was suggested that the accumulation of MUFA in LM relatively declined by increased genes.
Dietary factors and related metabolic interactions have direct and indirect nutrient influences on the regulation and expression of specific genes. It has been suggested that significant regulation occurs at the level of transcription, with controlled modulation of mRNA levels. It indicates that nutritional factors can also regulate gene expression by interacting with other regulatory networks depending on dietary fat. Other studies have demonstrated the regulation of apoprotein gene expression by a sucrose-rich diet, the nutritional regulation of gene expression in lipogenesis, and the suppression of fatty acid synthase transcription by PUFAs (Walker and Blackburn, 2004). PUFAs and hormones (leptin, adiponectin) that activate AMP-activated protein kinase have been reported to improve insulin sensitivity in rats (Suchankova et al., 2005).
As the result, it has been speculated these genes play an important role in the control of MUFA. Therefore, these results suggest that both genetic and nutritional factors should be considered for fatty acid composition in the LM. These results are due to the different expression of genes involved in insulin signaling pathway, but that will need to be confirmed by enzyme activity and protein function studies.
CONCLUSION
This study indicates that fat type had no significant effect on chemical composition and on meat quality as well as on growth performance in growing-finishing pigs. However, it could be manipulated by dietary nutrients because dietary fat type affected fatty acid composition in the LM contents. Especially, soybean and olive oil could be a substitute for beef tallow as commonly used fat additives. The six genes associated with insulin signaling pathway identified difference of the expression by microarray analysis. In particular, PRKAR2A and PPP1CB at the LM showed the highest expression level in the olive oil group. Expression of PRKAR2A and PPP1CB genes inversely matched with accumulation of MUFA in LM. Gene expression increased by absorbed a lot of MUFA, and it was suggested that the accumulation of MUFA in LM relatively declined by increased genes. The results of this study indicate that the type of dietary fat affects fatty acid composition and insulin signaling-related gene expression in the LM of pigs.
ACKNOWLEDGEMENTS
This study was supported by Agenda (200901OFT 072148173) of National Institute of Animal Science, Rural Development Administration, Republic of Korea.