Effect of Dietary L-ascorbic Acid (L-AA) on Production Performance, Egg Quality Traits and Fertility in Japanese Quail (Coturnix japonica) at Low Ambient Temperature
Article information
Abstract
Environmental stress boosts the levels of stress hormones and accelerates energy expenditure which subsequently imbalance the body’s homeostasis. L-ascorbic acid (L-AA) has been recognized to mitigate the negative impact of environmental stress on production performances in birds. The present investigation was carried out to elucidate the effect of different dietary levels of L-AA on production performance, egg quality traits and fertility in Japanese quail at low ambient temperature. Sixty matured females (15 wks) were equally divided into three groups (20/group) based on the different dietary levels of L-AA (0, 250 and 500 ppm) and coupled with an equal number of males (1:1) obtained from the same hatch. They were managed in uniform husbandry conditions without restriction of feed and water at 14 h photo-schedule. Except for feed efficiency, body weight change, feed consumption and hen-day egg production were recorded highest in 500 ppm L-AA supplemented groups. Among the all egg quality traits studied, only specific gravity, shell weight and thickness differed significantly (p<0.05) in the present study. Fertility was improved significantly (p<0.01) to a dose dependent manner of L-AA. The findings of the present study concluded that dietary L-AA can be a caring management practice at least in part to alleviate the adverse effect of cold induced stress on production performance in Japanese quail.
INTRODUCTION
Farm animals including birds are aware of thermal neutral zone in which the energy expenditure of the animals is minimal, constant and independent of environmental temperature. Preferably birds are naturally well adapted to cold due mainly to their highly efficient insulation provided by feathers (Ensminger et al., 1990). In spite of the nature’s adaptation, this creature is always being threatened by climatic, physical, parasitic and social stressors that elicit a reaction of the hypothalamic pituitary-adrenal (HPA) axis results in individual endocrine, immune and behavioral changes (McDowell, 1989; Dreiling et al., 1991; Leeson, 2001). Stressors principally stimulate glucocorticoids release which not only imbalance the antioxidant status (Klasing, 1998; Sahin et al., 2001a) but also reduce production performance, serum protein concentration and evidently augment oxidative stress. Sahin et al. (2002) pointed out that the level of plasma antioxidant vitamins (vitamin C, vitamin E, folic acid) and minerals also declined and oxidative damage increased according to the intensity of stressors. Low ambient temperature obliged cold stress that causes an increase in feed intake but decreased egg production and feed efficiency in laying hens (Ensminger et al., 1990; Spinu and Degen, 1993). Similar to heat stress, cold stress markedly increased serum corticosterone, blood glucose and cholesterol level (Donkoh, 1989; McDowell, 1989; Ensminger et al., 1990; Siegel, 1995) but concurrently decreased circulating level of protein, vitamins, minerals and antioxidant enzymes activities (Gumuslu et al., 2002). L-AA has been realized as an antioxidant vitamin and actively participates in mitigating the negative effect of stress on production performance. In spite of enough ability to in-vivo synthesis, to combat the stress effects birds need external supplementation for the maintenance of production performance and serum constituents such as glucose, urea, triglycerides, cholesterol, protein etc (Mowat, 1994; Bains, 1996; Kutlu and Forbes, 1999; Sahin et al., 2001a). Peebles and Brake (1985) reported the influence of ascorbic acid supplementation on the improvement of hatchability in broiler chickens. In addition, the vitamin was also accounted to have a positive impact on the fertility and hatchability in Pekin ducks (Kontecka et al., 2001) and pheasants (Nowaczewski and Kontecka, 2005).
However, limited information is available so far to correlate the negative impact of cold stress and its amelioration on production performance in Japanese quail. Therefore, the present study was an attempt to find out the effect of dietary L-AA on the production performance, egg quality and fertility in Japanese quail at low ambient temperature.
MATERIAL AND METHODS
Experimental design and diet
A total one hundred and twenty, sixty from each sex (15 wk old) Japanese quail from same hatch were procured from the institutional experimental quail farm. Females (randomly selected) were equally divided into three groups (I, II and III) and paired with males by 1:1 ratio. Each couple was maintained in individual cages (40×40×20 cm3) at 14 h photo-schedule. Birds from group-I were offered control diets (Table 1) whereas from other groups (II and III) obtained control diets eventually supplemented with L-AA (LC Industry Co. Ltd., Mumbai) by 250 ppm and 500 ppm respectively. Feed and water were ad-libitum to the birds from all the groups. During the course of experiment (6 wk), the ambient temperatures and relative humidity of the experimental shed were computed three times a day (06.00 h, 14.00 h and 22.00 h). Till the end of this experiment, the average ambient temperature and relative humidity inside the house were recorded 10.47±3.3°C and 69.35±2.7% respectively.
Performance variables and egg quality
Body weight and feed consumption was recorded at weekly interval throughout the experiment to determine the body weight changes and feed efficiency. To assess the hen-day egg production, eggs were collected everyday at the same time and the daily egg numbers were divided by the number of hens on that day. Egg weight was measured daily throughout the experimental period. Egg quality parameters such as specific gravity, albumen height, index and weight, yolk height, index and weight, Haugh unit, egg shell thickness, percentage and weight were evaluated weekly on the total eggs produced on a specific day. Specific gravity of eggs was determined by using the saline flotation method of Hempe et al. (1988). Salt solutions were made in incremental concentrations of 0.005 in the range from 1.065 to 1.120. The height of thick albumen and yolk were measured using Ames tripod stand micrometer. The average length and width of thick white and yolk were determined using a Vernier Caliper. Yolk weight was recorded only after complete separation from albumen even adhered albumen was also removed by rolling over a filter paper (No 1). Based on the albumen height and egg weight, Haugh unit was calculated following the formula described by Eisen et al. (1962). Once the eggs were broken onto a level surface, egg shells were carefully washed under running water, dried and weighted weekly in a digital electrical balance (Singh et al., 2009). The shell thickness was affirmed weekly by measuring thickness at three locations of each egg (air cell, equator, and sharp end) using a dial pipe gauge (Mitutoyo, 0.01–20 mm, Japan) and finalized by average of them.
Determination of percent fertility
Females were allowed to mate (1:1) naturally and egg collection was practiced twice daily from second day onwards till the end of experiment. Eggs were stored at low temperature (15–18°C) in the institute hatchery maximum a week before setting. Confirmation of fertility was made by breaking eggs at 7th day of incubation at 99.5°F temperature and 55% relative humidity. The percent fertility was calculated by the ratios of number of fertile eggs to the number of total egg set in the incubator.
Statistical analysis
Data generated during the course of experiment was analyzed by statistical software package (SPSS v16) for ANOVA (Snedecor and Cochran, 1994) and Duncan’s multiple range tests (Duncan, 1955) for comparing the mean values of control birds with other treatment groups.
RESULTS
The effect of dietary L-AA supplementation on production performance of laying Japanese quail hens at the low ambient temperature is shown in Table 2. Apart from feed efficiency, body weight change, feed consumption and hen-day egg production were significantly (p<0.05) higher in L-AA supplemented groups from the control. Further the change in body weight varied significantly (p<0.05) to the dose of L-AA. Feed consumption and hen-day egg production did not vary according to the dose of L-AA.
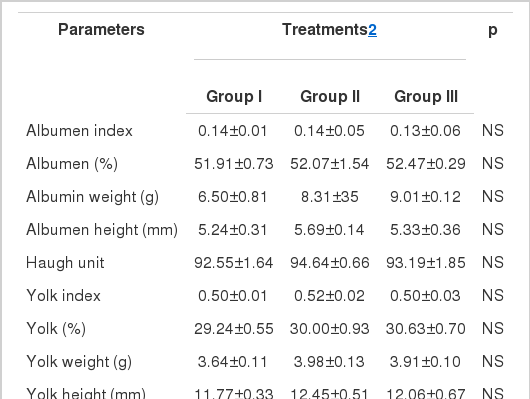
The mean values of egg quality (external and internal) traits recorded in all the groups under present study are shown in Table 3 and 4. Compared to control, significantly (p<0.05) higher specific gravity was recorded in L-AA supplemented groups. Although egg weight, shell % and shape index was improved with supplementation, statistically supplemented groups remained insignificant from the control group. Albumen and yolk quality traits (albumen index, percent, weight, height, yolk index, weight percent and height) were arbitrarily higher in the L-AA supplemented groups but their mean values did not vary from the control. Haugh unit did not change upon different treatments of L-AA. Egg shell of birds received highest concentration of L-AA did not have a thickness difference from the rest of the groups. It only differed from the control. The shell weight was found to be influenced by dietary L-AA supplementation. Thickest and heaviest egg shell was found in birds received highest concentration of L-AA and exhibited a significant (p<0.05) difference from the rest of the groups.
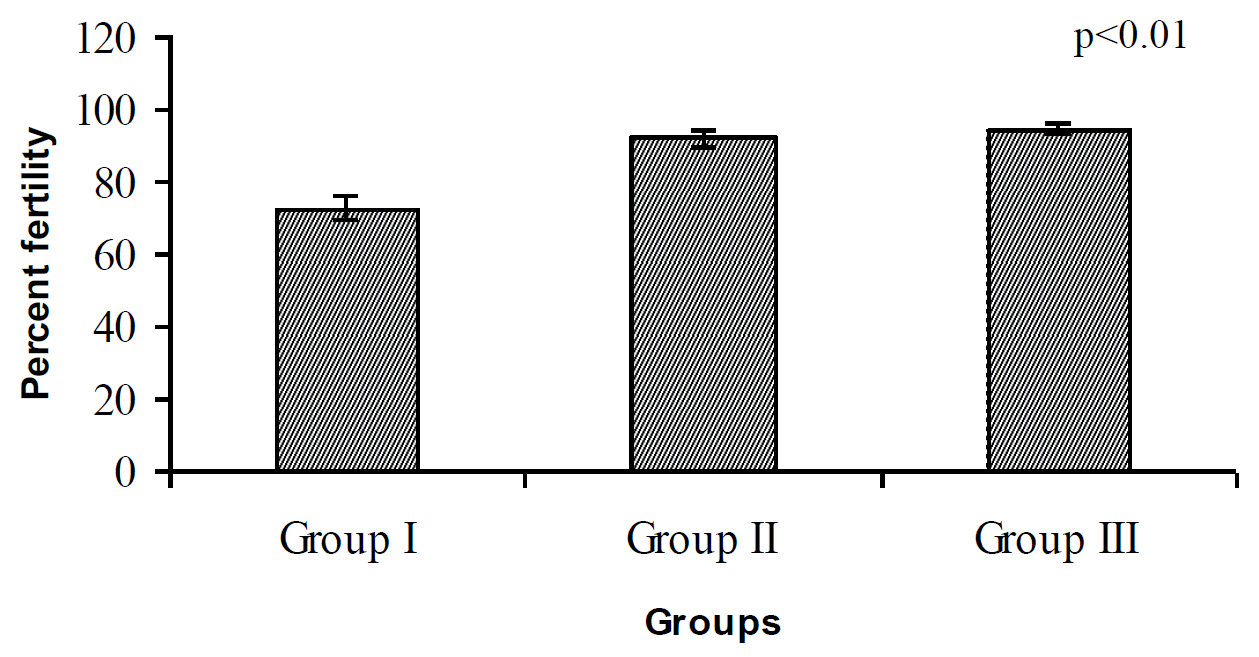
Fertility percent recorded in the present study is shown in Figure 1. Fertility of birds received highest concentration of L-AA did not differ from the rest of the groups. It only differed from the control.
DISCUSSION
The biosynthesis of ascorbic acid in the renal medulla and cortex of the birds can meet the physiological demands (Cheng et al., 1990), but temperatures above or below the thermo-neutral zone ensures its supplementation to prevent the alteration of physiological and biochemical constituents. As in other domesticated avian species, low ambient temperature has been found to induce stress that negatively affects production performance in laying Japanese quail.
In the current study, the highest body weight gain and daily feed intake was recorded in group III birds, which received highest concentration of L-AA (500 ppm), further the mean values were also differed significantly (p<0.05) from rest of the groups (Table 2). This result is in agreement to the Ensminger et al. (1990) and Arad and Marder (1982), who have reported that low ambient temperatures cause an increase in feed intake. Since the stress of low ambient temperatures cause an increase in feed intake, the further higher feed consumption upon L-AA supplementation is hard to be used as the indicator of amelioration of the negative effect of cold stress. Similar to all homeothermic animals, Japanese quail obviously needs to be maintained their core body temperature when exposed to the below thermo-neutral zone. Therefore, under such unfavorable conditions higher feed intake may be the probable cause to execute the demand of excess energy at low ambient temperature. Reportedly, body weight gain is positively correlated to the feed intake. Since the birds consumed more feed, resulting higher body weight gain which validated our observations. On contrary to our observations, several studies have speculated that dietary ascorbic supplementation has no effect on feed consumption or feed efficiency at environmental stress.
The hen-day egg production in L-AA supplemented groups was significantly (p<0.05) higher from the control group (Table 2) but remained unchanged between groups received increasing dietary L-AA. This present finding is in agreement to Kucuk et al. (2003) who have been reported beneficial effects of ascorbic acid on egg production at low ambient temperature. Njoku and Nwazota (1989) also ascertained that the ascorbic acid supplementation to laying hens in tropical conditions caused a proportional increase in the number of eggs laid. Birds received moderate concentration of ascorbic acid (250 ppm) showed highest mean egg weight but statistically no variation was drawn among the groups. The presence of antioxidant (vitamin C) could partially inhibit adversely oxidative protein denaturation and would improve nutrients digestibility and feed efficiency (Ciftici et al., 2005). This could be a reason in rising of layers performance.
The present study revealed a significant (p<0.01) difference in mean values of specific gravity between the groups (Table 3). The highest specific gravity was observed in L-AA supplemented groups compared to control group. That is meant that L-AA supplementation increased egg shell quality traits. Similar results were observed by Keshavarz (1996), Sahin and Sahin (2001b) and Kucuk et al. (2003). They demonstrated that egg specific gravity increased by vitamin C supplementation under normal and stress condition. The increase in specific gravity may be due to the increase in shell thickness. In the current study, the mean egg shell thickness and weight were found to be improved significantly (p<0.05) in accordance to increased dietary supplementation of L-AA concentration, which is a further proof of increased egg specific gravity. Further, shell thickness influences the egg shell weight, which is the cause of significant shell weight differences (p<0.05) recorded between the groups in the present study. In birds, L-AA augments conversion of 1,25 dihydoxycholecalciferol in the kidney and increases calcium mobilization from skeleton which suggests its role in egg shell formation and strength (Dorr and Balloun, 1976; Bollengier-Lee et al., 1999). Besides, the content of organic matrix in the shell is positively correlated to the available ascorbic acid. Therefore, these ascorbic acid associated consequences corroborate our current observations of increased egg shell thickness in dietary L-AA supplemented groups.
The internal egg quality parameters studied in the current experiment did not exhibit any significant change between groups (Table 4). Although the mean values of the said parameters were improved in accordance with dietary L-AA supplementation, however they remained unchanged throughout study. Our results are in agreement with the recent findings of Saki et al. (2010). However, as an ameliorative measure, several researchers have documented beneficial effects of ascorbic acid supplementation on egg quality traits in poultry when exposed to environmental stress (El-Boushy et al., 1988; Orban et al., 1993; Bains, 1996).
The fertility percentage recorded in the present study ranged from 72.5 to 94.57 where the highest (p<0.01) fertility was noticed in group III birds, which received maximum dietary L-AA concentration (Figure 1). Our observation is in agreement to Torgowski and Kontecka (1998), who reported higher egg fertility in the group of pheasants supplemented with 500 ppm L-AA. In addition dietary ascorbic supplementation upon stress influenced fertility and hatchability in broiler chickens (Peebles and Brake, 1985). Kontecka et al. (2001) also reported a positive impact of dietary ascorbic acid supplementation on the hatchability in Pekin ducks. The better results in terms of fertility estimated in the current study could also be an indirect effect of higher sperm viability and concentration resulting from the influence of dietary L-AA supplementation on male reproduction. The improvement in fertility may be due to its antioxidant effect that protects sperm from DNA damage, which is a major cause of compromised sperm function. It is now established that ascorbic acid is a powerful antioxidant because it can donate a hydrogen atom and form a relatively stable ascorbyl free radical. As a scavenger of reactive oxygen and nitrogen oxide species, ascorbic acid has been shown to be effective against the superoxide radical ion, hydrogen peroxide, the hydroxyl radical and singlet oxygen (Weber at al., 1996).
The results and ongoing discussion suggested that dietary L-AA supplementation alleviates the adverse effect of cold induced stress and improved the production performance in laying Japanese quail. Additionally, 500 ppm L-AA had been revealed more beneficial and could be practiced as protective management for the laying Japanese quail reared under low ambient temperature.