Antibacterial Activity of Recombinant Pig Intestinal Parasite Cecropin P4 Peptide Secreted from Pichia pastoris
Article information
Abstract
Cecropins (Cec) are antibacterial peptides and their expression is induced in a pig intestinal parasite Ascaris suum by bacterial infection. To explore the usefulness of its activity as an antibiotic, CecP4 cDNA was prepared and cloned into the pPICZ B expression vector and followed by the integration into AOX1 locus in Pichia pastoris. The supernatants from cell culture were collected after methanol induction and concentrated for the test of antimicrobial activity. The recombinant P. patoris having CecP4 showed antimicrobial activity when tested against Staphyllococcus aureus in disc diffusion assay. We selected one of the CecP4 clones (CecP4-2) and performed further studies with it. The growth of recombinant P. pastoris was optimized using various concentration of methanol, and it was found that 2% methanol in the culture induced more antibacterial activity, compared to 1% methanol. We extended the test of antimicrobial activity by applying the concentrated supernatant of CecP4 culture to Pseudomonas aeruginosa and E. coli respectively. Recombinant CecP4 also showed antimicrobial activity against both Pseudomona and E. coli, suggesting the broad spectrum of its antimicrobial activity. After improvements for the scale-up, it will be feasible to use recombinant CecP4 for supplementation to the feed to control microbial infections in young animals, such as piglets.
INTRODUCTION
Antibiotics have been used for treating infectious diseases, preventing diseases, and for livestock and poultry production as animal growth promoter (AGP) (Allen et al., 2013). But the concerns about the increase in the incidence of human infections by antibiotic-resistant bacteria and their spread led to bans on the use of the antibiotics as AGP for livestock production in Korea and European nations as well (Allen et al., 2013). Nonetheless, the use of antibiotics is allowed only for treatment purpose of prevention, control, and treatment of specific animal diseases. The bans on the use of antibiotics encourages the development of alternatives to antibiotics, including antimicrobial peptides (AMPs), probiotics, organic acids and vaccines, for food-producing animals (Allen et al., 2013). Among them, AMPs are peptides that are generated by the innate immune system and confer specific innate immunity against microbes. Various organisms, including microbes, plants and animals, produce AMPs as the first line of defense to fight against invading pathogenic microbes. AMPs possess broad-spectrum of bactericidal activity against Gram-positive bacteria, Gram-negative bacteria, fungi, parasites, virus and tumor cells (Bals, 2000). One of interesting features of AMPs is that they rarely elicit the resistance of bacteria that is a serious problem with conventional antibiotics (Lee et al., 1997). Therefore, AMPs have emerged as one of the most promising candidates for a new class of antibiotics (Hancock and Scott, 2000; Reddy et al., 2004).
Among AMPs, cecropins (Cec) are small (~4 kDa) peptides containing 35 to 39 amino acids, which are amphipathic, as a high proportion of basic amino acids are present at the N-terminus conferring a net positive charge and the hydrophobic amino acids are rich at the C-terminus (Sipos et al., 1992). Structural analyses revealed the formation of α-helix, which is split by a Glycine-Proline sequence in the well-studied cecropin A from H. cecropia (Steiner, 1982) and cecropin B2 from B. mori (Gazit et al., 1994). To date, four cecropins, namely cecropin P1–P4, had been reported in Ascaris suum which is a parasite found in gut of pig (Pillai et al., 2005). All of them consist of 31 amino acids forming α-helix throughout the entire structure and disc diffusion assay using synthetic Cec peptides showed broad-spectrum of antibacterial activity against various pathogenic bacteria (Pillai et al., 2005). These findings suggest that Cec might be highly potent and effective novel antibiotics. But the cost of producing CecPs by using solid phase chemical synthesis could restrict their practical use as alternatives for antibiotics. Therefore, production of antimicrobial peptides using a microorganism could be an efficient alternative. In this regard, Pichia pastoris, which is a methylotrophic yeast, can be an attractive host for the expression of recombinant protein, as it has been developed as an excellent host for the high level expression of heterologous proteins under the AOX1 (alcohol oxidase1) gene promoter which is tightly regulated by methanol (Cereghino and Cregg, 2000; Cregg et al., 2000; Macauley-Patrick et al., 2005). Therefore, we sought to develop Pichia pastoris expression system to express recombinant CecP4 and test its antimicrobial activity against pathogenic bacteria for livestocks. The present study shows the successful manipulation of CecP4 for expression in Pichia pastoris and the secretion of a functional AMP to inhibit pathogenic bacteria which may impact livestock health.
MATERIALS AND METHODS
Cloning of CecP4 cDNA
To get the CecP4 cDNA, we used RT-PCR technique using isolated total RNA from Ascaris suum. Extraction of total RNA was conducted by using the TRI reagent according to the manufacturer’s instruction. Isolated total RNA (500 ng) was reverse transcribed using 5 U of AMV reverse transcriptase XL for 30 min at 42°C. The prepared cDNA was amplified by PCR using the following primers: CecP4-1S, 5′-GCGCGCGAATTCCTACTCGTCATGTTT GC -3′; CecP4-1AS, 5′-GCGCGCTCTAGATATAATTGTT CCCGTAC -3′. The PCR condition was as follows; one cycle of 60 s at 94°C, 35 cycles each for 30 s at 94°C, 30 s at 58°C and 30 s at 72°C, and one cycle of 10 min at 72°C. PCR products were cloned into pGEM-T (Promega, WI, USA) vector and the sequence was verified.
Subcloning into pPICZB vector and screening
The PCR product of the full length ORF of Ascaris CecP4 was cleaved with EcoRI/XbaI restriction enzymes (NEB, USA) and was cloned into the P. pastoris expression vector pPICZB of EasySelect Pichia Expression Kit (Invitrogen, CA, USA). The resulting vector (pPICZB::CecP4) contained the full length ORF of CecP4 at downstream of the secretion signal sequence of Saccharomyces cerevisiae α-factor prepropeptide and ZeocinTM resistance gene as a selection marker. In order to integrate the pPICZB::CecP4 plasmid into the 5′-AOX1 region of P. pastoris (X33), we linearized the pPICZB::CecPs with SacI restriction enzyme (NEB). Transformation was performed by the lithium chloride method according to the manufacturer’s protocols (Invitrogen, USA). To determine whether the pPICZB::CecP4 plasmid integrated into P. pastoris, individual colonies were picked and analyze for the presence of pPICZB::CecP4 plasmid as well as a control transformant (C) with empty vector (pPICZB). After the genomic DNAs were isolated by the manufacturer’s protocols (Invitrogen), the presence of the integrated CecP4 was confirmed by PCR using the specific primers of AOX1 gene: forward primer; 5′-GACTGGTTCCAATTGACAA GC-3′/reverse primer; 5′-GCAAATGGCATTCTGACA TCC-3′.
Induction of recombinant CecP4 peptide using methanol
To determine if the transformed cells were able to secrete the recombinant CecP4 as an active form with antibacterial activity, the transformants and control (C) were grown in 20 mL of MM (Minimal methanol; 1.34% yeast nitrogen base (YNB), 1% methanol, 400 μg/L biotin) media at 30°C for 96 h. The cell culture supernatant was collected by removing the pellet after centrifuging at 500 g rpm for 5 min.
Disc diffusion assay
The supernatants which were concentrated after methanol induction were subjected to the antibacterial activity assay by determining the size of clear zones, i.e., growth inhibition zone against the lawn of bacterial strains tested; Staphylococcus aureus KCTC 1621, Pseudomonas aeruginosa and Escherichia coli, respectively. These bacteria were either purchased from the KRIBB Gene Bank or kindly provided by Dr. Choi Kang Duk at Han-Kyong Nat’l University. Bacterial culture was grown to early log phase (OD at 600 nm ≈0.5, corresponding to 1~2×108/mL CFU) and then poured on the test media, followed by placing a cylindrical plastic tube (6 mm inner diameter×8 mm high) on top of the lawn of bacteria culture. Thereafter, 180 μL of the supernatant was loaded inside the tube. The plates were incubated at 37°C overnight and the size of the clear zone around each cylindrical tube was observed.
RESULTS
Cloning of Ascaris CecP4 cDNA into pPICZB P. pastoris expression vector and screening
The 267 bp fragment of full length CecP4 ORF cloned into pPICZ B vector (267 bp+3,329 bp = 3,596 bp) which is verified by restriction enzyme (Figure 1A) and sequencing. The expected expression of the peptide is shown in Figure 1B. Once we integrated the vector into the 5′-AOX1 region of Pichia pastoris (X33) and confirmed it by PCR, we try to find transformants which has antibiotic activity. Among the many clones, clone #2 (CecP4-2) showed strong antibacterial activity against Staphylococcus aureus KCTC 1621. After then, we used it for further study.
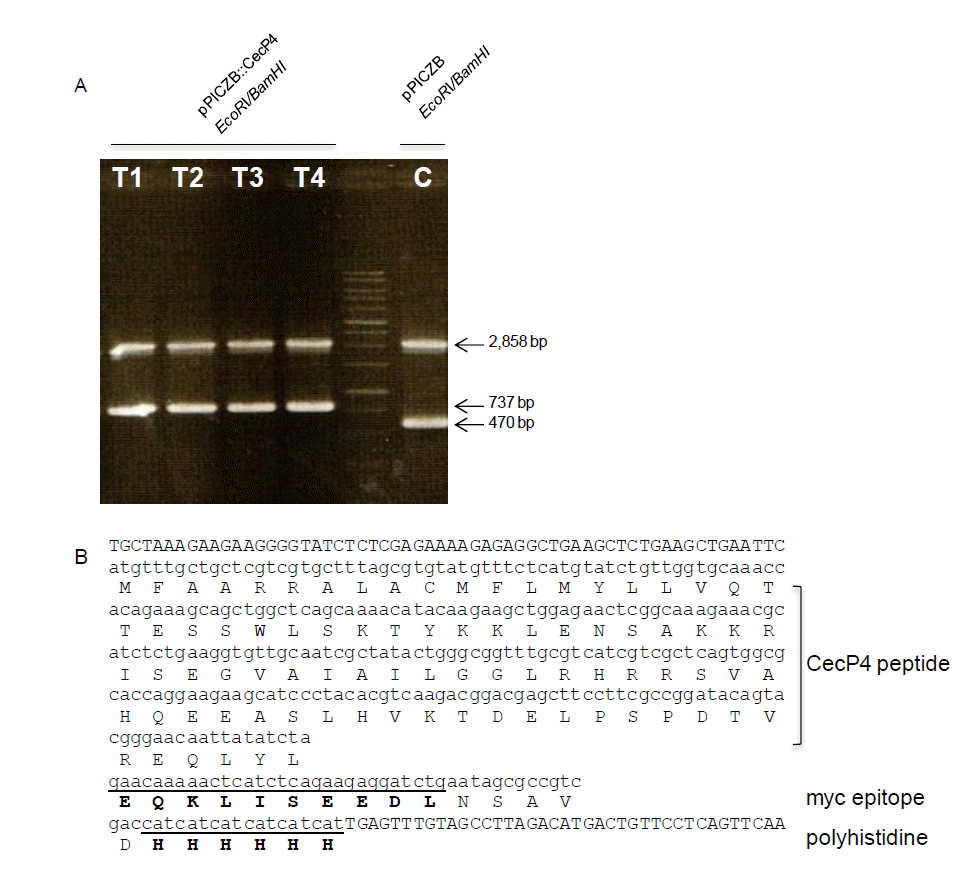
Identification of the integrated pPICZA::CecP4 recombinant plasmid into Pichia pastoris AOX1 gene. (A) Identification of integrated pPICZA::CecP4 by restriction enzyme digestion. Genomic DNAs were isolated from control strain with empty vector, pPICZA (C) and four transformants with pPICZA::CecP4 (T1, T2, T3, and T4) and used for restriction enzyme digestion with EcoRI and Xba I. (B) Sequences analysis of the insert CecP4 in pPICZA::CecP4. Amino acids with bold letters represent myc epitope and polyhistidine, respectively.
Recombinant Ascaris CecP4 peptide expression condition
We investigated several different methanol concentrations to establish the culture conditions for the highest production of CecP4. The clone CecP4-2 was cultured in 20 mL of MMY media at 30°C, 200 rpm for 120 h. Different volumes of 100% methanol were added into the cultures to a final concentration of 1% and 2% every day. Samples were harvested every 24 h and the antibacterial activity of CecP4 was determined by agar diffusion assay. As shown in Figure 2A, the growth rates of the control strain (empty vector) and transformant (CecP4-2) were dependent on methanol concentration of the culture media. The growth of the transformant was higher in 1% methanol than in 2% at 48 h (Figure 2B). The antibacterial activity of the CecP4-2 reached up to its maximum after 72 h incubation with 2% methanol, maintaining activity to 120 h, and to a lesser extent at the 72 h and 96 h time points with 1% methanol concentration (Figure 2B). Based on the results, the antibacterial activity of recombinant CecP4 seems to be correlated with the growth of the strain, but not directly proportional.
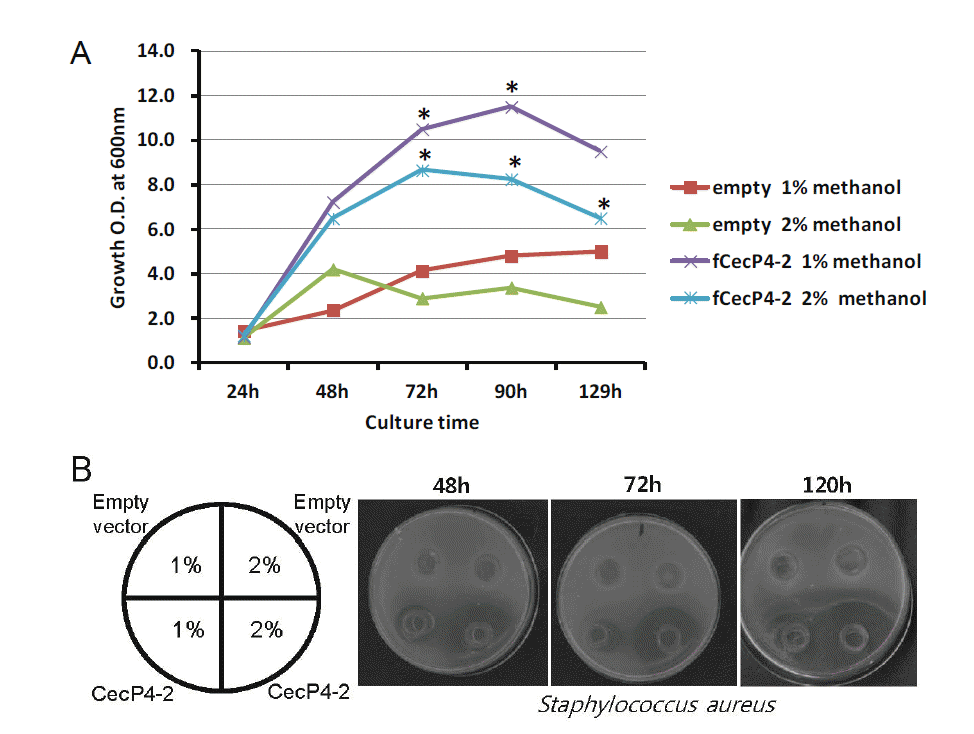
Growth curve of the CecP4 transformant and antibacterial activity of secreted recombinant CecP4 from P. pastoris. (A) Growth curve of CecP4 transformant with different dose of methanol for induction. For these experiments, CecP4 transformant (CecP4-2) and control transformant were grown in different concentrations of methanol media (1% and 2%) for the indicated time before measuring the cell growth by spectrometric analysis of the culture density. This is a representative result of two experiments (n = 2, * p<0.05). (B) Antibacterial activity of secreted recombinant CecP4 protein against Staphylococcus aureus KCTC 1621. Antibacterial activity was analyzed by agar diffusion assay using the culture supernatants for the indicated time points.
Antibacterial activity of secreted recombinant CecP4 on pathogenic bacteria
We found that concentrated supernatant of recombinant CecP4 maintained bactericidal activity toward Staphylococcus aureus (Figure 3A). In addition, when tested against other pathogenic microbes, i.e., Pseudomonas aeruginosa and Esherichia coli, concentrated recombinant CecP4 exhibited comparable antibacterial activity with gentamycin (Pseudomonas aeruginosa: Figure 3B, Esherichia coli: Figure 3C, respectively), demonstrating that recombinant CecP4 produced from Pichia pastoris might possess the bactericidal activity toward broad spectrum of pathogenic microbes. Further study warrants to apply Pichia pastoris produced recombinant CecP4 toward other pathogenic microbes.
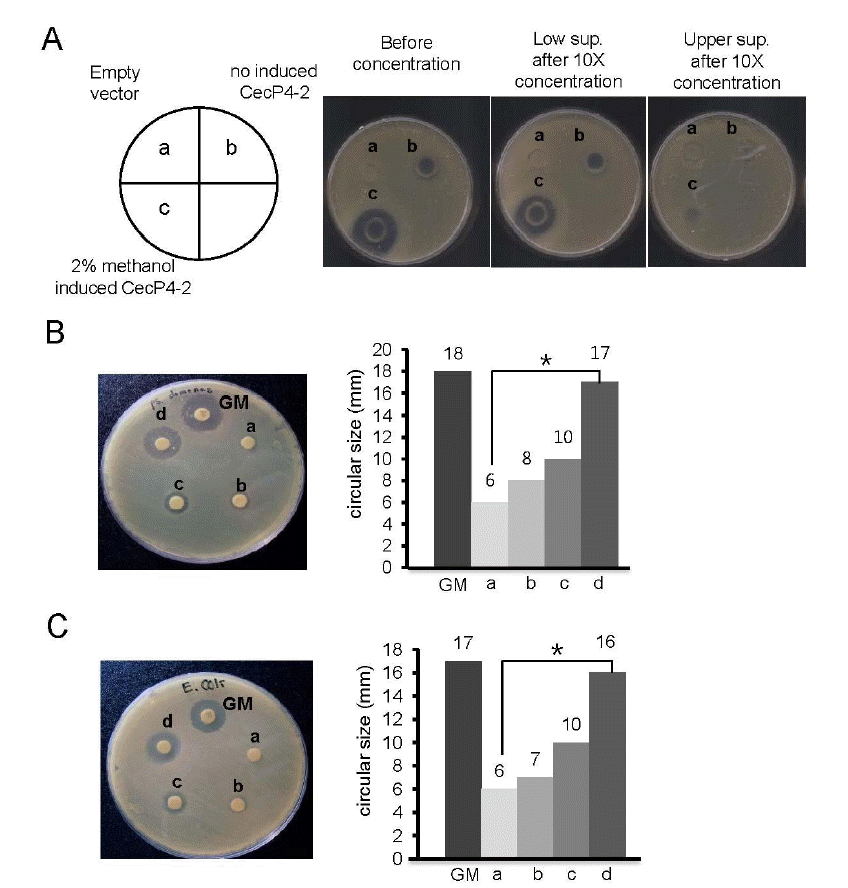
Antibacterial activity of concentrated recombinant CecP4 secreted from P. pastoris using disc diffusion assay. (A) Antibacterial activity of concentrated recombinant CecP4 peptide against S. aureus. Culture supernatants were concentrated and upper and lower supernatant after concentration were used for disc diffusion assay, respectively. a: empty vector, b: supernatant (lower), c: supernatant (upper) (This is a representative result of three experiments (n = 3, * p<0.05). (B) Antibacterial activity of concentrated recombinant CecP4 on Pseudomonas. Pseudomonas were treated with different dose of concentrated recombinant CecP4 and incubated 37°C for 18 h. This is a representative result of three experiments (n = 3, * p<0.05). Concentrated supernatant of recombinant CecP4 (10×), a: 25 μL, b: 50 μL, c: 100 μL, d: 200 μL. GM: gentamycin 10 μL (10 mg/mL). (C) Antibacterial activity of concentrated recombinant CecP4 on Escherichia coli. Escherichia coli were treated with different dose of concentrated recombinant CecP4 and incubated 37°C for 18 h. This is a representative result of three experiments (n = 3, * p<0.05). Concentrated supernatant of recombinant CecP4 (10×), a: 25 μL, b: 50 μL, c: 100 μL, d: 200 μL. GM: gentamycin 10 μL (10 mg/mL).
DISCUSSION
Cecropin is the antimicrobial peptide family which was initially found in pig intestine (Lee et al., 1989), but later it turned out that CecP1 and other related family peptides, i.e., CecP2, CecP3, and CecP4, were expressed in pig intestinal parasite Aascaris suum, more specifically body wall, uterus, ovary, and intestine in response to bacterial infection (Pillai et al., 2005). Screening in the pig and mammalian cDNA libraries failed to identify the homologous sequences to CecPs, suggesting that CecPs were present exclusively in A. suum genome (Philla et al., 2005). All CecPs were bactericidal against Gram-positive such as Staplyococcus aureus, Bacillus subtilis, Micrococcus luteus, and Gram-negative bacteria, including Pesudomonas aeruginosa, Salmonella typhimurium and E. coli (Pillai et al., 2005).
The expression of secreted recombinant proteins in Pichia pastoris offers several advantages over bacterial expression systems. These include appropriate folding of molecules and disulfide bond formation, as well as execution of post-translational modifications which conserve protein function. Secretion of recombinant proteins circumvents intracellular accumulation, a significant aspect in the expression of toxic proteins. The secretion of recombinant proteins also simplifies their purification by avoiding contamination with intracellular proteins (Damasceno et al., 2004; Daly and Hearn, 2005). These advantages make secreted recombinant protein production in Pichia pastoris popular for scientific research (Guo et al., 2012).
In conclusion, the present study demonstrates the successful manipulation of CecP4 for expression in yeast and the secretion of a functional AMP. Further studies are warranted to optimize the culture condition for mass production of recombinant CecP4 and to test antimicrobial activity against broad spectrum of pathogens from chicken and pigs for the practical application of secreted recombinant CecP4 to livestock production as a feed supplement.
ACKNOWLEDGEMENT
This work was supported by the Next Generation of BioGreen Project 21 (PJ0008196), RDA, Republic of Korea and Inha University Research Grant.