 |
 |
Abstract
Somatic cell nuclear transfer (SCNT) is a useful tool for animal cloning, but the efficiency of producing viable offspring by SCNT is very low. To improve this efficiency in the production of cloned pigs, it is critical to understand the interactions between uterine function and cloned embryos during implantation. Lysophosphatidic acid (LPA) is a lipid mediator that plays an important role in the establishment of pregnancy in pigs; however, LPA production in the uterine endometrium of pigs carrying SCNT-cloned conceptuses has not been determined. Therefore, we investigated expression of ENPP2, an LPA-generating enzyme, in the uterine endometrium of gilts with conceptuses derived from SCNT during the implantation period. Uterine endometrial tissue and uterine flushing were obtained from gilts carrying SCNT-derived conceptuses and from gilts carrying conceptuses resulting from natural mating on d 12 of pregnancy. Our results demonstrated no difference in the level of ENPP2 mRNA expression in the uterine endometrium between gilts carrying SCNT-derived conceptuses and gilts carrying naturally-conceived conceptuses, but secretion of ENPP2 protein into the uterine lumen did decrease significantly in pigs with SCNT-derived conceptuses. These results indicate that expression and secretion of ENPP2, which are critical for appropriate LPA production and successful pregnancy, are dysregulated in the uterine endometrium of pigs carrying SCNT-derived conceptuses.
Embryo implantation, a physical and physiological connection between the conceptus and the uterine endometrium, is accompanied by appropriate conceptus-uterine interaction (Tranguch et al., 2005). In pigs, approximately 30% of conceptuses die between d 12 and 30 of pregnancy, underscoring the importance of the implantation process for the establishment and maintenance of pregnancy (Pope et al., 1986; Pope, 1988). A variety of signaling molecules, including steroid hormones, growth factors, adhesion molecules, and cytokines, are involved in this process (Bazer et al., 1998; Spencer and Bazer, 2004). Among these molecules, the role of lysophosphatidic acid (LPA) has recently become a topic of particular interest.
LPA is a lysophospholipid with various biological functions that are mediated through at least six specific receptors, LPAR1-6 (Gardell et al., 2006). Increasing evidence indicates that LPA plays an important role in the establishment and maintenance of pregnancy in various animal species (Ye et al., 2005; Seo et al., 2008; Liszewska et al., 2009; Seo et al., 2012). Mice without the Lpar3 gene showed reproductive defects such as uneven embryo spacing and delayed implantation, which is associated with decreased prostaglandin (PG) production (Ye et al., 2005). In sheep, LPA has been found in uterine flushings, and LPA treatment increases cell proliferation and production of PGE2 and PGF2Îą in trophectoderm cells (Liszewska et al., 2009). LPAR3 in pigs is expressed in the uterine endometrium and levels of LPAR3 expression increase on day 12 of pregnancy, when the conceptus begins to implant; LPA also increases endometrial PTGS2 expression (Seo et al., 2008).
The LPA structure consists of a glycerol backbone with a fatty acyl chain and a free phosphate group (Ishii et al., 2004). LPA has structurally diverse forms due to variation in the length and saturation of the fatty acyl side chain (Gerrard et al., 1989; Tigyi and Miledi, 1992). In the porcine uterus, several LPA species are present in the luminal fluid, and the levels of some LPA species in the uterine luminal fluids are higher on d 12 of pregnancy than on d 12 of the estrous cycle (Seo et al., 2008), suggesting that the pregnancy status regulates LPA production in the uterine lumen differentially.
Extracellular LPA is generated from membrane phospholipid by sequential actions of enzymes including phospholipase A1 and A2 (PLA1/PLA2) and lysophospholipase D (lysoPLD) (Aoki et al., 2008). PLA1 or PLA2 cuts fatty acyl chains bound to the phospholipids, generating lysophospholipid (LPL). Then, lysoPLD remove the head group of LPL to convert it to LPA. It is thought that ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2) is extracellularly secreted lysoPLD, which generates LPA from lysophospholipids, mainly lysophosphatidylcholine (Aoki et al., 2008). Previously, we showed that uterine endometrium and conceptus produce ENPP2 and secrete it into the uterine lumen in pigs (Seo et al., 2012). The levels of ENPP2 protein and lysoPLD activity were higher in the uterine lumen on d 12 of pregnancy than on d 12 of the estrous cycle, which is associated with increased levels of some LPA species in the uterine lumen on d 12 of pregnancy (Seo et al., 2008; Seo et al., 2012). These findings suggest that ENPP2 has a role in the establishment of pregnancy by regulating LPA production in the uterine lumen during the implantation period in the pigs.
Somatic cell nuclear transfer (SCNT) is a useful technique to clone animals, but the efficiency of producing viable offspring by SCNT is very low (Campbell et al., 2005). Inappropriate reciprocal interactions between the implanting conceptuses derived from SCNT and the maternal uterus are one of the reasons for high pregnancy failure in cloned animal gestation (Bauersachs et al., 2009; Mansouri-Attia et al., 2009). A variety of signaling molecules, including estrogen, interleukin-1β (IL1B), and LPA, are involved in the conceptus-uterine interaction during the implantation period in pigs (Burghardt et al., 1997; Bazer et al., 1998; Seo et al., 2008). To improve the low efficiency in production of cloned pigs, it is necessary to understand the effects of these signaling molecules in the uterus in SNCT-cloned pregnancy. Since we have shown that many endometrial genes are aberrantly expressed in the uterine endometrium of pigs carrying SCNT-derived conceptuses (Ka et al., 2008; Kim et al., 2009), we hypothesized that expression of ENPP2 might also change in the uterus of pigs carrying SCNT-derived conceptuses, which can affect LPA levels and, in turn, the establishment of pregnancy in pigs. Therefore, in this study, we analyzed ENPP2 expression in the uterine endometrium and levels of ENPP2 protein in the uterine lumen in pigs carrying SCNT-derived conceptuses.
All experimental procedures involving animals were conducted in accordance with the Guide for Care and Use of Research Animals in Teaching and Research and approved by the Institutional Animal Care and Use Committee of Yonsei University. Gilts were hysterectomized on d 12 of pregnancy. Pregnancy was confirmed by the presence of apparently normal conceptuses with filamentous morphology in uterine flushings. Endometrium dissected from the myometrium was collected from two different areas of the middle portion of each uterine horn. Uterine flushings from d 12 of pregnancy were obtained by introducing and recovering 50 mL phosphate-buffered saline (PBS, pH 7.4) at hysterectomy (25 mL/uterine horn). The uterine flushings were clarified by centrifugation (3,000Ăg for 10 min at 4°C), aliquoted, and frozen at â80°C until analysis. Endometrial tissues, dissected free from the myometrium, were collected from two different areas of the middle portion of each uterine horn, snap-frozen in liquid nitrogen, and stored at â80°C for RNA and protein extraction. For immunohistochemistry, cross-sections of endometria were fixed in 4% paraformaldehyde in PBS (pH 7.4) for 24 h and then embedded in paraffin as previously described (Ka et al., 2000).
Endometrial tissue samples from four gilts carrying embryos generated by SCNT on d 12 of pregnancy were obtained as described previously (Ka et al., 2008; Kim et al., 2009). Ovoidal conceptuses were recovered from the uteri of four pigs with SCNT-derived embryos. Uterine tissues from gilts carrying conceptuses resulting from natural mating and from gilts carrying embryos generated by SCNT were classified as Non-NT and NT, respectively.
Total RNA was extracted from endometrial tissues using TRIzol reagent (Invitrogen Life Technology, Carlsbad, CA) according to the manufacturerâs recommendations. The quantity of RNA was assessed spectrophotometrically, and the integrity was validated by electrophoresis on a 1% agarose gel. Four micrograms of total RNA were treated with DNase I (Promega, Madison, WI) and reverse-transcribed using SuperScript II Reverse Transcriptase (Invitrogen) to obtain cDNA.
To analyze levels of ENPP2 mRNAs in the uterine endometrium, real-time RT-PCR was performed using the Applied Biosystems StepOnePlus System (Applied Biosystems, Foster City, CA) with the SYBR Green method. Complementary DNAs were synthesized from 4 Îźg of total RNA isolated from different uterine endometrial tissues. Newly synthesized cDNAs (21 ÎźL) were diluted 1:4 with sterile water and then used for PCR. Specific primers based on porcine ENPP2 (GenBank accession number XM_003125509, forward, 5â˛-CAA GAC AAA ATC AAA CAG TAT GTG G-3â˛; reverse, 5â˛-CTT CTA CCC ACT TGG ACT CAT CTT-3â˛) and porcine ribosomal protein L7 (RPL7), (GenBank accession number NM_001113217; forward, 5â˛-AAG CCA AGC ACT ATC ACA AGG AAT ACA-3â˛; reverse, 5â˛-TGC AAC ACC TTT CTG ACC TTT GG-3â˛) were designed to amplify 205-bp and 172-bp fragments, respectively, from the cDNA. The Power SYBR Green PCR Master Mix (Applied Biosystems) was used for PCR reactions. The final 20 ÎźL reaction volume included 2 ÎźL of cDNA, 10 ÎźL of 2X Master mix, 2 ÎźL of each primer, and 4 ÎźL of dH2O. PCR conditions were 95°C for 10 min followed by 40 cycles of 95°C for 15 s, 60°C for 35 s, and 72°C for 45 s. The results are reported as the expression level relative to the levels on d 12 of the estrous cycle after normalizing to the endogenous RPL7 control by the 2âÎÎCT method (Livak and Schmittgen, 2001).
Endometrial tissues were homogenized in lysis buffer (1% Triton X-100, 0.5% Nonidet P-40, 150 mM NaCl, 10 mM Tris, 1 mM EDTA, 0.2 mM Na3VO3, 0.2 M PMSF, and 0.5 Îźg/mL NaF) at a ratio of 100 mg tissue per 1 mL buffer, and the cellular debris was removed by centrifugation (16,500Ăg for 5 min). The protein concentration in endometrial lysates and uterine flushings was determined using a Bradford protein assay (Bio-Rad Laboratories, Richmond, CA) with BSA as the standard. Twenty micrograms of endometrial protein lysate or 10 Îźg protein from uterine flushings was loaded in each lane and electrophoresed on 12% SDS-PAGE gels followed by electrotransfer onto nitrocellulose membranes. Nonspecific binding was blocked with 5% (w/v) fat-free milk in Tris-buffered saline with 0.1% (v/v) Tween-20 (TBST) buffer for 1 h at room temperature. The blot was incubated overnight at 4°C with 0.5 Îźg/mL of rabbit polyclonal anti-ENPP2 antibody diluted in 2% milk/TBST, which detects the carboxy-terminus of the ENPP2 protein (CosmoBio, Tokyo, Japan). The blot was washed in TBST at room temperature three times for 10 min each, incubated with peroxidase-conjugated goat anti-rabbit secondary antibody (1:20,000; Jackson Laboratories, West Grove, PA) for 1 h at room temperature, and rinsed again for 30 min at room temperature with TBST. Immunoreactive proteins were detected by chemiluminescence (SuperSignal West Pico, Pierce Chemical Co, Rockford, IL) according to the manufacturerâs recommendations using X-ray films (Agfa-Gevaert). Blots were reblotted with rabbit polyclonal anti-β-actin (ACTB) antibody (1:5,000; Sigma) to assess consistent loading. The integrated optical density of ENPP2 and ACTB bands in the immunoblots was quantified by scanning densitometry using an HP1210 (HP, Seoul, Korea) and GelPro Analyzer (Media Cybernetics, Silver Spring, MD). Values are presented as the ratio of each ENPP2 signal to the corresponding ACTB signal.
To determine which cells in the porcine endometrium express ENPP2, sections were immunostained. Sections (5 Îźm thick) were deparaffinized and rehydrated in an alcohol gradient. Antigen retrieval was performed by boiling tissue sections in citrate buffer (pH 6.0) for 10 min. Tissue sections were washed with PBS with 0.1% (v/v) Tween-20 (PBST) three times and blocked with 0.5% (v/v) H2O2 in methanol for 30 min. Tissue sections were then blocked with 10% normal goat serum for 30 min at room temperature. Rabbit polyclonal anti-ENPP2 antibody (1 Îźg/mL, CosmoBio) was added and incubated overnight at 4°C in a humidified chamber. For each tissue tested, purified normal rabbit IgG was substituted for the primary antibody as a negative control. Tissue sections were washed with PBST three times. Biotinylated goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA) was added and incubated for 1 h at room temperature. Following washes with PBST, a streptavidin peroxidase conjugate (Invitrogen) was added to the tissue sections, which were then incubated for 10 min at room temperature. The sections were washed with PBST, and aminoethyl carbozole substrate (Invitrogen) was added to the tissue sections, which were then incubated for 10 min at room temperature. The tissue sections were washed in water, counterstained with Mayerâs hematoxylin, and coverslipped.
Previously, we have shown that ENPP2 is expressed in the uterine endometrium in pigs (Seo et al., 2012). In this study, to determine whether ENPP2 is normally expressed in uterine endometrium carrying SCNT-derived conceptuses during the implantation period, we compared levels of ENPP2 mRNAs in gilts carrying SCNT-derived conceptuses with gilts carrying Non-NT conceptuses on d 12 of pregnancy. Real-time RT-PCR analysis showed that ENPP2 mRNA levels were not different between Non-NT and NT (p>0.05; Figure 1).
Next, we compared ENPP2 protein levels between Non-NT and NT. Immunoblot analysis showed a major band with a molecular weight (MW) of 50,000 (Figure 2A), as shown previously (Seo et al., 2012). Although ENPP2 mRNA levels were not different between Non-NT and NT (Figure 1), levels of ENPP2 protein with a MW of 50,000 on d 12 of pregnancy were significantly higher in NT than Non-NT (p<0.01; Figure 2B).
Since it has been shown that endometrial ENPP2 protein with MW of 20,000 is secreted into the uterine lumen in pigs (Seo et al., 2012), we determined whether the uterine endometrium of gilts carrying SCNT-derived conceptuses secretes ENPP2 protein into the uterine lumen. Total proteins from uterine flushings obtained from Non-NT and NT on d 12 of pregnancy were analyzed by immunoblotting. Immunoblot analysis showed that secreted ENPP2 protein was detected only in uterine flushings of Non-NT, and not of NT (Figure 3).
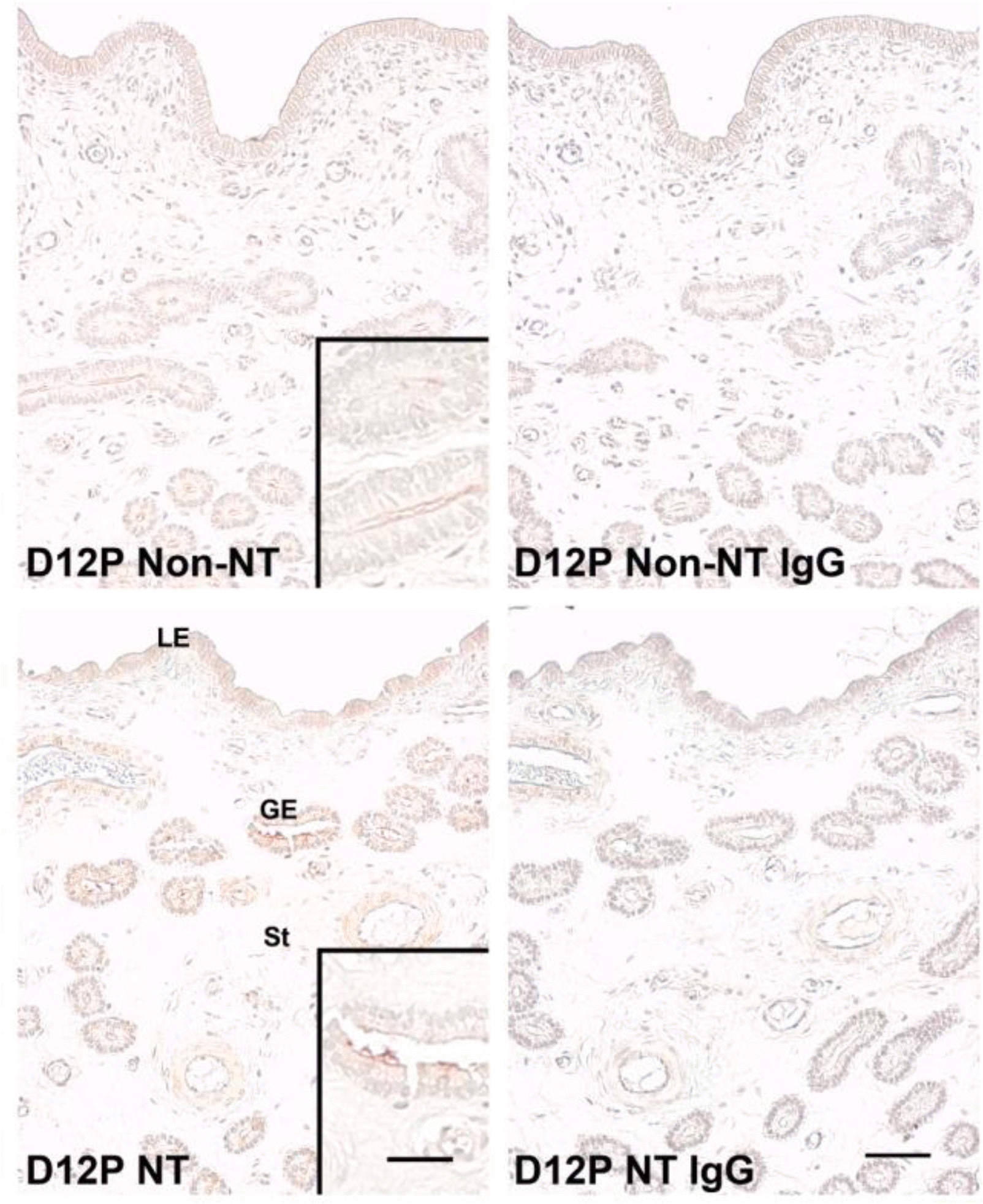
Next, we used immunohistochemistry to analyze localization of protein in the porcine uterine endometrium from Non-NT and NT on d 12 of pregnancy. ENPP2 protein was localized primarily to the LE and GE in the endometrium from both Non-NT and NT on d 12 of pregnancy, with a stronger staining signal intensity in NT (Figure 4).
The novel findings of this study are that i) ENPP2 mRNA levels in the uterine endometrium are not affected by SCNT-derived conceptuses on d 12 of pregnancy; ii) levels of ENPP2 protein increased in the uterine LE and GE of gilts carrying SCNT-derived conceptuses on d 12 of pregnancy; and iii) the uterine endometrium with SCNT-derived conceptuses did not secrete ENPP2 protein into the uterine lumen on d 12 of pregnancy. To our knowledge, this is the first report investigating the effect of SCNT-derived conceptuses on ENPP2 expression in the uterine endometrium during the implantation period in pigs.
LPA has diverse biological functions, which are mediated and regulated by LPA receptors, LPAR1-6 (Ishii et al., 2004). Among these receptors, LPAR3 seems to be a key receptor for conceptus implantation in pigs because LPAR3 expression sharply increases in the uterine endometrium on d 12 of pregnancy, when conceptus implantation begins, and LPA upregulates PTGS2 expression through LPAR3 (Seo et al., 2008). Accumulating evidence indicates that LPA action is also regulated by LPA level. LPA-generating activity is different depending on pregnancy status, gestation stage, and pathological conditions, showing the higher activity in the serum of pregnant women than non-pregnant women and late gestation stage than early stage (Tokumura et al., 2002a; Tokumura et al., 2002b; Tokumura et al., 2009). In pigs, levels of some LPA species in the uterine lumen are much higher on d 12 of pregnancy than d 12 of the estrous cycle (Seo et al., 2008), suggesting that LPA production in the uterine lumen is regulated by the presence of the conceptus. Extracellular LPA is produced from LPL by action of ENPP2 with lysoPLD activity (Aoki et al., 2008). Thus, to understand the regulation of LPA production at the maternal-conceptus interface in pigs, it is essential to determine expression of ENPP2 in the uterus. Previously, we found that ENPP2 is expressed in the uterine endometrium and secreted into the uterine lumen in pigs. The levels of secreted ENPP2 protein are higher in the uterine lumen on d 12 of pregnancy than on d 12 of the estrous cycle, which is associated with increased production of some LPA species in the uterine luminal fluids (Seo et al., 2008; Seo et al., 2012). This suggests that ENPP2 plays an important role in the establishment of pregnancy in pigs by regulating LPA production at the maternal-conceptus interface.
The use of SCNT is a valuable technique for cloning various animal species including pigs, but the survival rate of SCNT-cloned embryos is very low (Lee et al., 2004; Wells, 2005). The high rate of pregnancy failure is associated with abnormal placental development (Kim et al., 2005; Chae et al., 2006; Jouneau et al., 2006). Recent studies have shown that expression of endometrial genes in response to SCNT-cloned embryos is greatly altered at the time of implantation in cows (Bauersachs et al., 2009; Mansouri-Attia et al., 2009), indicating that abnormal placental development in the pregnancy of a cloned embryo may be due to inappropriate embryo-maternal communication during the implantation period. In this study, ENPP2 mRNA levels in the uterine endometrium were not different between gilts with SCNT-derived conceptuses and gilts with conceptuses derived from natural mating, while ENPP2 protein levels were much higher in the uterine endometrium of gilts carrying SCNT-derived conceptuses. Interestingly, however, secretion of ENPP2 protein was not detectable in the uterine lumen of pigs carrying SCNT-derived conceptuses. We speculate that lower ENPP2 protein levels in the uterine lumen from the uterus with SCNT-derived conceptuses than those with conceptuses from natural mating is caused by insufficiency of embryonic signals to activate endometrial function related to ENPP2 secretion or activation in the uterus with SCNT-derived conceptuses. Indeed, our preliminary studies have shown that levels of estrogen and IL1B, which are major embryonic signals at the time of pregnancy in pigs (Bazer et al., 1998), are significantly lower in the uterine lumen with SCNT-derived conceptuses than those with conceptuses from natural mating (Seo and Ka, unpublished results). Nevertheless, these results indicate that the secretory mechanism of ENPP2 protein is impaired in the endometrium with SCNT-derived conceptuses, and lack of secreted ENPP2 protein, in turn, may result in insufficient LPA production in the uterine lumen causing pregnancy failure in pigs with SCNT-cloned embryos.
Porcine conceptuses secrete a variety of molecules such as estrogen, IL1B, and interferon-Îł during the peri-implantation period (Jaeger et al., 2001). In response to conceptus-secreting molecules, the uterine endometrium also secretes hormones, protease inhibitors, growth factors, enzymes, transport proteins, and extracellular matrix for the establishment of pregnancy and conceptus development (Geisert and Yelich, 1997). Thus, we hypothesized that the signaling pathways involved in the embryo-maternal communications are impaired in the uterine endometrium with SCNT-cloned conceptuses. Indeed, our recent data indicate that estrogen and IL1B levels are decreased in the uterine lumen in the endometrium with SCNT-derived conceptuses (Seo and Ka, unpublished results). Furthermore, we have shown that expression of LPAR3, an estrogen-responsive gene (Seo et al., 2008), and SAL1, an IL1B-responsive gene (Seo et al., 2011), decreases in the uterine endometrium of pigs carrying SCNT-derived conceptuses during the implantation period (Seo and Ka, 2011). These findings suggest that both IL1B and estrogen signaling mechanisms are impaired in the uterine endometrium in pigs carrying SCNT-derived conceptuses. Although estrogen and IL1B do not directly increase ENPP2 expression in the uterine endometrium (Seo and Ka, unpublished data), it is likely that inappropriate estrogen and IL1B signals between the endometrium and the SCNT-derived conceptuses lead to insufficient secretion of ENPP2 by the uterine endometrium. Further study is still needed to investigate the factors of conceptuss involved in the secretion of ENPP2.
In summary, we found that the uterine endometrium in pigs carrying SCNT-derived conceptuses does not secrete ENPP2 protein normally into the uterine lumen during the implantation period. Our results suggest that appropriate expression and secretion of ENPP2 and LPA production may be critical for the establishment of a successful pregnancy for SCNT-derived conceptus in pigs.
ACKNOWLEDGMENTS
This study was supported the Next Generation BioGreen 21 Program (#PJ009610), Rural Development Administration, and the National Research Foundation Grant (NRF-2010-413-B00024) funded by the Korean Government.
Figure 1
Real-time RT-PCR analysis of ENPP2 mRNA expression in the uterine endometrium carrying conceptuses derived from SCNT (NT) or from natural mating (Non-NT) in pigs. Endometrial tissue samples from gilts on d 12 of pregnancy that carried SCNT embryos (n = 4) and gilts with embryos resulting from natural mating (n = 6) were analyzed. Abundance of mRNA is presented as the expression relative to the level of ENPP2 mRNA in the uterine endometrium of gilts with Non-NT conceptuses after normalization of the transcript amount to RPL7. Data are presented as means with SEM.

Figure 2
Immunoblot analysis of ENPP2 protein in the uterine endometrium carrying conceptuses derived from SCNT (NT) or from natural mating (Non-NT) in pigs. (A) Endometrial tissues from gilts on d 12 of pregnancy that carried NT embryos (n = 4) and gilts with embryos resulting from Non-NT (n = 4) were tested. ENPP2 bands were detected with an approximate molecular weight of 50,000. ACTB was used as a loading control. (B) The ratio of ENPP2 protein optical density to ACTB density was measured by scanning densitometry. Data are presented as means with SEM.
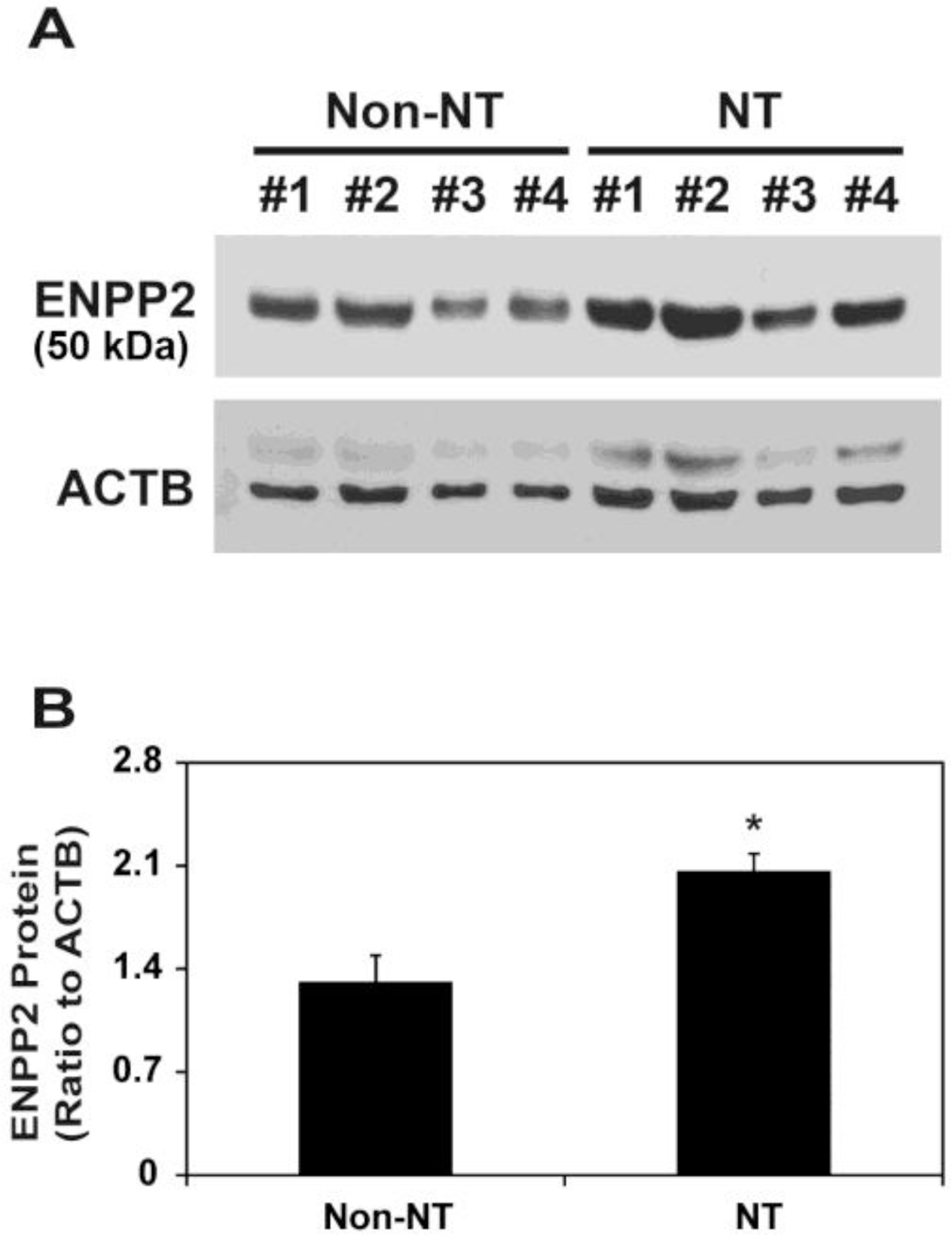
Figure 3
Analysis of ENPP2 protein levels in uterine flushings from pigs by immunoblot analysis. Uterine flushings were obtained from gilts on d 12 of pregnancy that carried SCNT embryos (NT) and gilts with embryos resulting from natural mating (Non-NT), and ENPP2 protein levels were measured by immunoblot analysis. (A) ENPP2 bands were detected with an approximate molecular weight of 20,000. (B) Densitometry of ENPP2 in uterine flushings was measured by scanning densitometry. Data are presented as mean optical density with SEM.
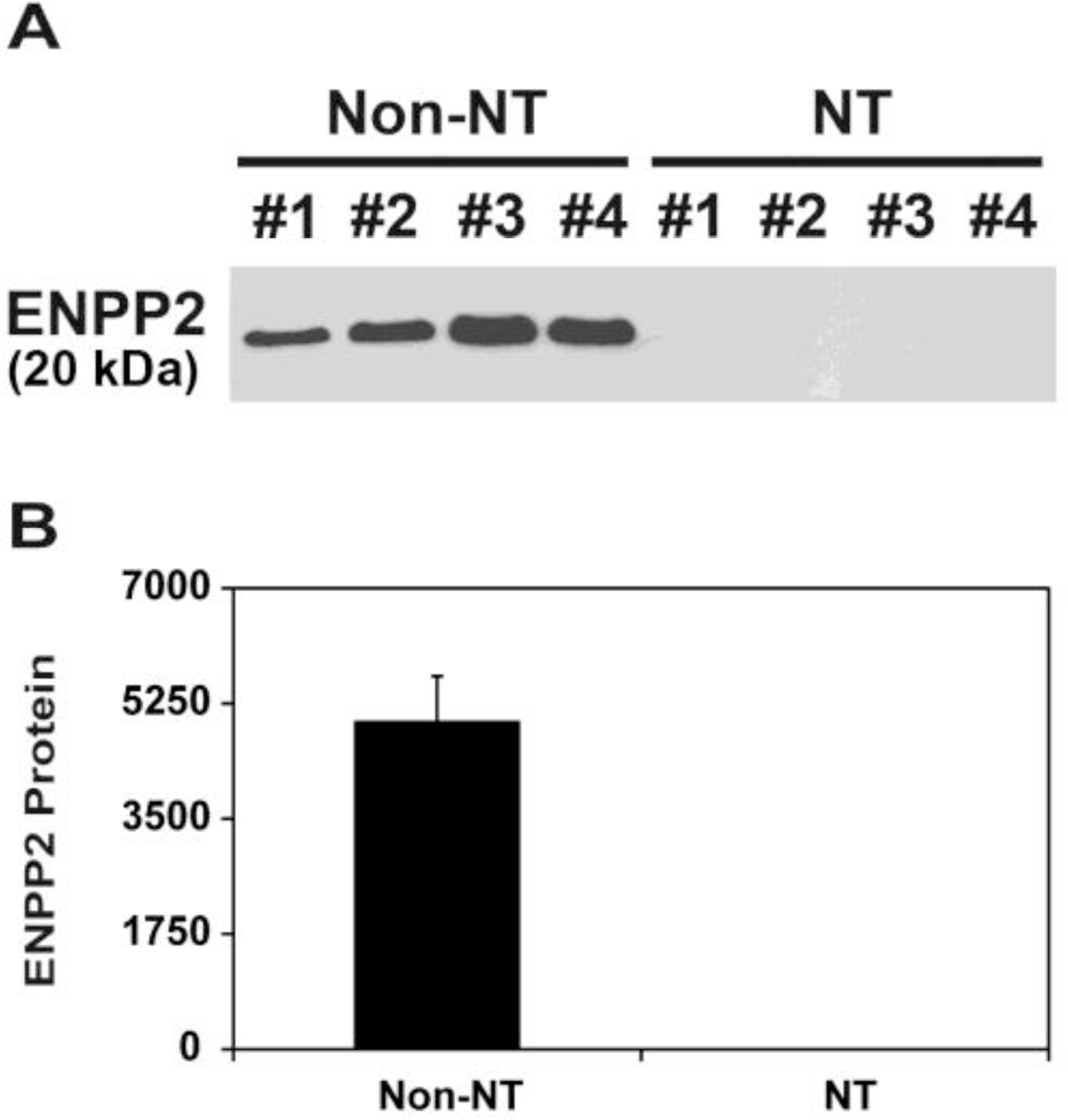
Figure 4
Immunohistochemistry of ENPP2 protein in the porcine uterine endometrium carrying conceptuses derived from SCNT (NT) or from natural mating (Non-NT) on d 12 of pregnancy in pigs. Immunoreactive ENPP2 protein shown in brown was localized primarily to LE and GE. Uterine sections immunostained with normal rabbit IgG were shown as a negative control. D = Day; P = Pregnancy; LE = Luminal epithelial cells; GE = Glandular epithelial cells; St = Stroma. Scale bar = 50 Îźm and 25 Îźm in inset.
REFERENCES
Aoki J, Inoue A, Okudaira S. 2008. Two pathways for lysophosphatidic acid production. Biochim. Biophys Acta 1781:513â518.


Bazer FW, Ott TL, Spencer TE. 1998. Endocrinology of the transition from recurring estrous cycles to establishment of pregnancy in subprimate mammals. Endocrinology of pregnancy. Bazer FW, editorTotowa, NJ: Humana Press; p. 1â34.

Bauersachs S, Ulbrich SE, Zakhartchenko V, Minten M, Reichenbach M, Reichenbach HD, Blum H, Spencer TE, Wolf E. 2009. The endometrium responds differently to cloned versus fertilized embryos. Proc. Natl. Acad. Sci USA 106:5681â5686.



Burghardt RC, Bowen JA, Newton GR, Bazer FW. 1997. Extracellular matrix and the implantation cascade in pigs. J. Reprod. Fertil Suppl 52:151â164.


Campbell KH, Alberio R, Choi I, Fisher P, Kelly RD, Lee JH, Maalouf W. 2005. Cloning: eight years after Dolly. Reprod Domest Anim 40:256â268.


Chae JI, Cho SK, Seo JW, Yoon TS, Lee KS, Kim JH, Lee KK, Han YM, Yu K. 2006. Proteomic analysis of the extraembryonic tissue from cloned porcine embryos. Mol. Cell Proteomics 5:1559â1566.


Gardell SE, Dubin AE, Chun J. 2006. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med 12:65â75.


Geisert RD, Yelich JV. 1997. Regulation of conceptus development and attachment in pigs. J. Reprod. Fertil Suppl 52:133â149.


Gerrard JM, Robinson P. 1989. Identification of the molecular species of lysophosphatidic acid produced when platelets are stimulated by thrombin. Biochim. Biophys Acta 1001:282â285.


Ishii I, Fukushima N, Ye X, Chun J. 2004. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem 73:321â354.


Jaeger LA, Johnson GA, Ka H, Garlow JG, Burghardt RC, Spencer TE, Bazer FW. 2001. Functional analysis of autocrine and paracrine signalling at the uterine-conceptus interface in pigs. Reprod Suppl 58:191â207.


Jouneau A, Zhou Q, Camus A, Brochard V, Maulny L, Collignon J, Renard JP. 2006. Developmental abnormalities of NT mouse embryos appear early after implantation. Development 133:1597â1607.


Ka H, Seo H, Kim M, Moon S, Kim H, Lee CK. 2008. Gene expression profiling of the uterus with embryos cloned by somatic cell nuclear transfer on day 30 of pregnancy. Anim Reprod Sci 108:79â91.


Ka H, Spencer TE, Johnson GA, Bazer FW. 2000. Keratinocyte growth factor: expression by endometrial epithelia of the porcine uterus. Biol Reprod 62:1772â1778.


Kim HR, Kang JK, Yoon JT, Seong HH, Jung JK, Lee HM, Park CS, Jin DI. 2005. Protein profiles of bovine placenta derived from somatic cell nuclear transfer. Proteomics 5:4264â4273.


Kim M, Seo H, Choi Y, Hwang W, Lee CK, Ka H. 2009. Aberrant expression of retinol-binding protein, osteopontin and fibroblast growth factor 7 in the porcine uterine endometrium of pregnant recipients carrying embryos produced by somatic cell nuclear transfer. Anim Reprod Sci 112:172â181.


Lee RS, Peterson AJ, Donnison MJ, Ravelich S, Ledgard AM, Li N, Oliver JE, Miller AL, Tucker FC, Breier B, Wells DN. 2004. Cloned cattle fetuses with the same nuclear genetics are more variable than contemporary half-siblings resulting from artificial insemination and exhibit fetal and placental growth deregulation even in the first trimester. Biol Reprod 70:1â11.


Liszewska E, Reinaud P, Billon-Denis E, Dubois O, Robin P, Charpigny G. 2009. Lysophosphatidic acid signaling during embryo development in sheep: involvement in prostaglandin synthesis. Endocrinology 150:422â434.


Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(âDelta Delta C(T)) method. Methods 25:402â408.


Mansouri-Attia N, Sandra O, Aubert J, Degrelle S, Everts RE, Giraud-Delville C, Heyman Y, Galio L, Hue I, Yang X, Tian XC, Lewin HA, Renard JP. 2009. Endometrium as an early sensor of in vitro embryo manipulation technologies. Proc. Natl. Acad. Sci USA 106:5687â5692.



Pope WF, Lawyer MS, Nara BS, First NL. 1986. Effect of asynchronous superinduction on embryo survival and range of blastocyst development in swine. Biol Reprod 35:133â137.


Seo H, Ka H. 2011. Expression of lysophosphatidic acid receptor 3 in the uterine endometrium of pigs with somatic cell nuclear transfer cloned conceptuses. J Anim Sci Technol 53:203â209.

Seo H, Kim M, Choi Y, Lee CK, Ka H. 2008. Analysis of lysophosphatidic acid (LPA) receptor and LPA-induced endometrial prostaglandin-endoperoxide synthase 2 expression in the porcine uterus. Endocrinology 149:6166â6175.


Seo H, Choi Y, Shim J, Kim M, Ka H. 2012. Analysis of the lysophosphatidic acid-generating enzyme ENPP2 in the uterus during pregnancy in pigs. Biol Reprod 87:77


Seo H, Choi Y, Shim J, Choi Y, Ka H. 2012. Regulatory mechanism for expression of IL1B receptors in the uterine endometrium and effects of IL1B on prostaglandin synthetic enzymes during the implantation period in pigs. Biol Reprod 87:31


Spencer TE, Bazer FW. 2004. Uterine and placental factors regulating conceptus growth in domestic animals. J. Anim. Sci 82:E-SupplE4â13.

Tigyi G, Miledi R. 1992. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem 267:21360â21367.


Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. 2002. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem 277:39436â39442.


Tokumura A, Kanaya Y, Miyake M, Yamano S, Irahara M, Fukuzawa K. 2002. Increased production of bioactive lysophosphatidic acid by serum lysophospholipase D in human pregnancy. Biol Reprod 67:1386â1392.


Tokumura A, Kume T, Taira S, Yasuda K, Kanzaki H. 2009. Altered activity of lysophospholipase D, which produces bioactive lysophosphatidic acid and choline, in serum from women with pathological pregnancy. Mol Hum Reprod 15:301â310.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





