Effects of Sheng Hua Tang on Uterine Involution and Ovarian Activity in Postpartum Dairy Cows
Article information
Abstract
The effects of Sheng Hua Tang (SHT) on uterine involution and ovarian activity were investigated in postpartum dairy cows. SHT (70 g) was given to dairy cows (n = 10) to evaluate its effects for five days from the first postpartum day. Postpartum cows fed with a basal diet without SHT were used as the control group (n = 10). Ultrasounds and blood tests were recorded for four weeks from postpartum day seven with a 3-d interval. The results showed that the areas and diameters of endometria were significantly (p<0.01) reduced in the group that received SHT compared to the control group on the seventh postpartum day. The group that received SHT had an intrauterine fluid volume mean of 1.2±0.6 cm3, which was significantly lower than that of the control group, 2.3±0.8 cm3 (p<0.01) on the 13th postpartum day. In addition, the uterine tension score was a mean of 1.0±0.0 in the group that received SHT, which was also significantly lower than that of the control group, 1.5±0.5 (p<0.01) on the 19th postpartum day. Taken together, the Chinese herbal medicine remedy, SHT, promoted uterine involution and ovarian activity in postpartum dairy cows.
INTRODUCTION
Chinese herbal medicines have been used by postpartum nurses, in humans, for hundreds of years. The ancient Chinese book noted that delivery of a baby exhausts a woman’s physical health and that Sheng Hua Tang (SHT) helps to promote blood flow, resolve blood stasis, warm the meridians/channels, alleviate pain, and eliminate lochia (Bensky and Barolet, 1993). Chinese women commonly use SHT after giving birth. In Taiwan, more than 80% of women take SHT postpartum (Chuang et al., 2009). The formula of SHT consists of five ingredients including: Radix Angelicae Sinensis (Danggui), Ligustici Rhizoma (Chuan Xiong), Semen Persicae (Tao Ren), Zingiberis Rhizoma (Pao Jiang), and Glycyrrhizae Radix (Zhi Gan Cao). Radix Angelicae Sinensis is the major ingredient of SHT. Angelicae polysaccharide isolated from Radix Angelicae Sinensis may directly or indirectly stimulate the bioactivity of bone marrow macrophages in the hematopoietic inductive microenvironment to accelerate the synthesis and secretion of hematopoietic regulation factors at the gene and protein levels (Wang and Zhu, 1996; Wang et al., 2004; Li and Wang, 2005). These beneficial hematopoietic effects may also be mediated by the anti-inflammatory properties of Radix Angelicae Sinensis (Hu et al., 1991).
In dairy cows, reproductive problems usually occur during calving and post parturition. Reestablishment of postpartum reproductive efficiency involves the resumption of normal ovarian activity and uterine involution. Uterine involution involves the contraction of the uterus, sloughing of the caruncles and regeneration of the endometrium (Gier and Marion, 1968). Complete uterine involution can be observed when the size of the uterus shrinks to its pre-gravid state (Hussain and Daniel, 1991). In previous reports, rectal ultrasonography was successfully used to evaluate uterine involution in postpartum dairy cows (Ginther, 1998). The recovery of endometrial areas as well as scored intrauterine fluid volumes was used for indexing of uterine involution (Hu et al., 1991; Mateus et al., 2002). The first ovarian cycle after parturition is shorter than the normal oestrous cycle in cows; the first postpartum ovulation occurs 17 to 34 d after calving in most dairy cows (Kindahl et al., 1999). The first ovulation is generally at 19±1 d postpartum in dairy cows milked twice daily, and the earliest time to ovulation is 10 to 15 d after parturition (Youngquist, 1997). Early postpartum ovulation and pregnancy within three estrus cycles can improve the fertility of dairy cows (Thatcher and Wilcox, 1973; Benmrad and Stevenson, 1986). The appearance of the first significant progesterone rise (≥1 ng/mL) indicates the resumption of ovarian activity (Stevenson, 1997; Opsomer et al., 2000). In many reports of endometritis and uterine infections, lutein serves as the target of active ovarian response (Peter and Bosum, 1987; Gilbert et al., 1990; Huszenicza et al., 2005).
In most dairy cows, uterine involution is completed 4 to 5 wks postpartum; the earliest time for uterine involution is about three weeks postpartum. If the fetal membranes are retained and/or endometritis develops in the uterus of a cow, the time to uterine involution can be prolonged for 30 to 50 d (Lindell et al., 1982). Because a large volume of necrotic tissue is produced in a fluid medium (lochia) during the process of normal involution, the postpartum uterine cavity can be contaminated by bacteria. Bacteria can be naturally cleared from the uterus in the first two to three weeks after calving in most healthy cows (Bondurant, 1999). If dairy cows are severely infected with bacteria, antibiotics and hormone-prepared drugs can be used for treatment. However, the problems of drug retention and resistance are concerns. In many reports, lutein was used for the treatment of endometritis and uterineinfections (Peter and Bosu, 1987; Gilbert et al., 1990; Huszenicza et al., 2005).
In this study, SHT was used to treat the uterine diseases of postpartum dairy cows in China. The scientific evidence supporting SHT activity is still limited. In this investigation, ultrasonography was used to study the uterus and blood testing to determine the concentration of plasma progesterone to evaluate the effects of SHT on the process of uterine involution and ovarian activity in postpartum dairy cows.
MATERIALS AND METHODS
Experimental animals
Twenty Holstein-Friesian cows, 2 to 8 yrs old (average body weight 586 kg; 1st lactation: n = 4, 2nd lactation: n = 6, 3rd lactation: n = 6, and 4th lactation: n = 4), were used in this study. The cows did not receive any treatment during the month prior to the beginning of the experiment. These cows were housed in the animal shed at the farm of Hsinchu Branch Station, COA-TLI, Hsinchu, Taiwan, under identical environmental conditions. The use of animals was approved by the Institutional Animal Care and Use Committee (IACUC). All cows were normally calved without periparturient diseases, such as dystocia, uterine prolapse and retained fetal membranes, and housed in free-stalls.
Sheng Hua Tang treatment
According to our pretrial results showed that cows fed with 30 g (n = 3) or 50 g (n = 3) of SHT powder for five days no positive effects in uterine involution of postpartum cows. However, it showed positive effects on cows fed with 70 g (n = 3) of SHT powder (data not shown). So, in this study, 20 lactating postpartum Holstein-Friesian cows were randomly assigned to 2 groups, control (n = 10, fed with basal diet) and treatment (n = 10, fed with basal diet and SHT herb powder, 70 g per d). The 70 g of SHT powder (Koda Pharmaceutical Ltd., Taoyuan, Taiwan) was dissolved in 300 mL water for feeding. During the dry period (30 d of prepartum) cows were fed with 4 kg concentrate and 4 kg alfalfa hay daily, and Bermuda hay was accessed freely. After calving, the amount of concentrate fed was based on the milking level using a 1:3 concentrate to milk ratio (upper limit was 10 kg of concentrate). The concentrate composition is shown in Table 1. Alfalfa hay, supplied for roughage, had an upper limit of 8 kg, while Bermuda hay was accessed freely. The nutrient supply followed NRC (1989). Mineral salt and water were supply ad libitum. The individuals of the treatment group orally received SHT for five days starting from the postpartum d 1 to d 5.
Ultrasonography
The reproductive organs of cows were examined for 4 wks from the postpartum d 7 at a 3-d interval by the same veterinarian. Ultrasonography was conducted by a 5 MHz transrectal linear transducer (SonoSite® TITAN™, SonoSite, Inc., USA) to assess uterine characteristics of the gravid and nongravid horns. Diameters and areas of the endometria at the base of each uterine horn (approximately 5 cm anterior the uterine body) were measured and recorded by cross-sectional images (Okano and Tomizuka, 1987). If the structure of the scanned images was not spherical, the diameters of two 90° dimensions of an uterine horn were averaged to obtain the diameter values (Sheldon et al., 2002). The intrauterine fluid volumes were scored in a 0 to 3 scale: 0 represents no fluid and 1 to 3 represent the increasing fluid volumes in the uterine hornsas the previously described (Mateus et al., 2002). The uterine tonicities were scored in a 1 to 3 scale: 1 represents the uteruses with high tonicity, 2 represents the uteruses with moderate tonicity and 3 represents the flaccid uteruses (Melendez et al., 2004). The presences of luteal tissues in gravid and ipsilateral ovaries were also recorded by ultrasonography.
Progesterone assay
Blood samples were collected following the procedure described previously (Paape et al., 1972) with modifications. Briefly, 9 mL of blood were collected aseptically from the jugular vein using an 18 gauge needle into a vacuum K3 EDTA tube (Becton Dickinson Vacutainer System, Franklin Lakes, NJ, USA) as aforementioned periods. The tubes were centrifuged at 2,200×g for 10 min to harvest the plasma and stored at −20°C until it was analyzed for progesterone. Progesterone was measured in duplicate for each sample with a hormonal chemiluminescence assay (CIA) (Galbreath, 2008) by Union Clinical Laboratory, Taipei. The measuring range is 0.5 to 60 ng/mL.
Statistical analysis
The results were presented as mean±SEM. Statistical comparisons between control and experiment groups were performed using student’s t test for the endometrial area, diameter of horn, intrauterine fluid volume and uterine tension score. The appearance of the first significant progesterone rise (≥1 ng/mL) indicating the resumption of ovarian activity. Wilcoxon rank sum test was used for two-group comparisons of days of ovulation postpartum. A p-value of 0.05 or less was considered statistically significant (SAS, 2002).
RESULTS
Effects of Sheng Hua Tang treatment on the areas and diameters of the endometria
The diameters of two 90° dimensions of the uterine horns, a spot to b spot, was the minor axis, and c spot to d spot was the major axis, Figure 1; they were measured and averaged to obtain values for the diameters. The average endometrial area of the gravid horns, in the SHT-treated cows, was approximately 8.3 cm smaller (p<0.01) than that of the control group on day seven postpartum (Figure 2A). The endometrial diameters of the gravid horns were also reduced significantly on day seven (p<0.01) and d 16 postpartum (p<0.05) in the SHT-treated group (Figure 3A). However, the endometrial areas and diameters of the non-gravid horns had no significant differences in both experimental and control groups (Figure 2B, and Figure 3B). The results showed that treatment with SHT led to earlier uterine recovery in the cows.

Ultrasonogram of endometria in dairy cows. Diameters and areas of the endometria at the base of each uterine horn (approximately 5 cm anterior the uterine body) were measured and recorded by cross-sectional images. Note: If the structure of the scanned images was not spherical, the diameters of two 90° dimensions (a spot to b spot is the minor axis, c spot to d spot is the major axis) of uterine horns were averaged to obtain the values of diameters.
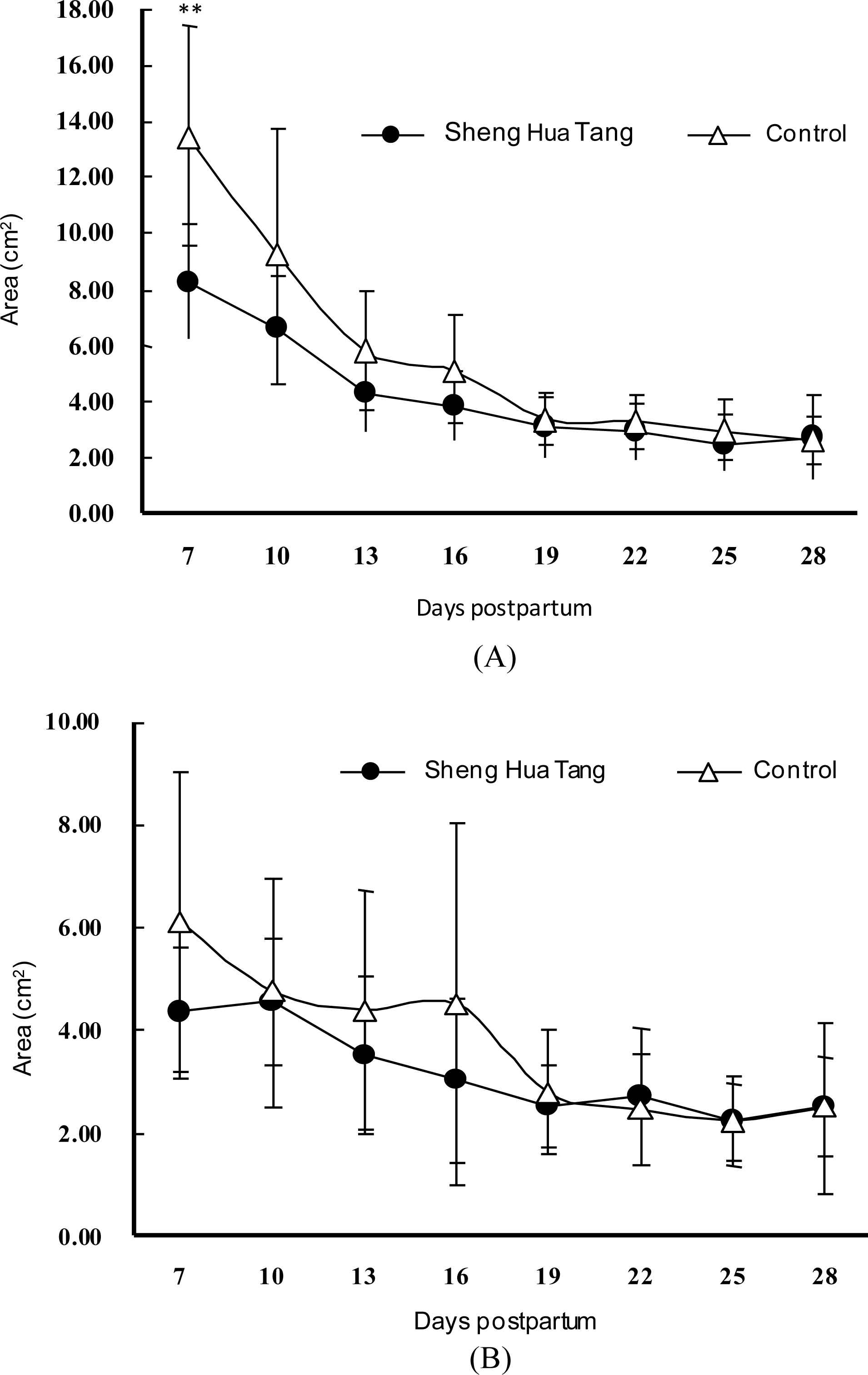
Effects of Sheng Hua Tang treatment on involutionary progress curves for endometrial areas of uterine horn in postpartum dairy cows. Means±SE (n = 10). (A) The endometrial area of gravid uterine horn was obviously reduced in the Sheng Hua Tang-receiving group than the control group on the 7th d postpartum. ** Means differ significantly between groups (p<0.01); (B) The endometrial area of nongravid uterine horn, there were no significant differences in both experimental and control groups.
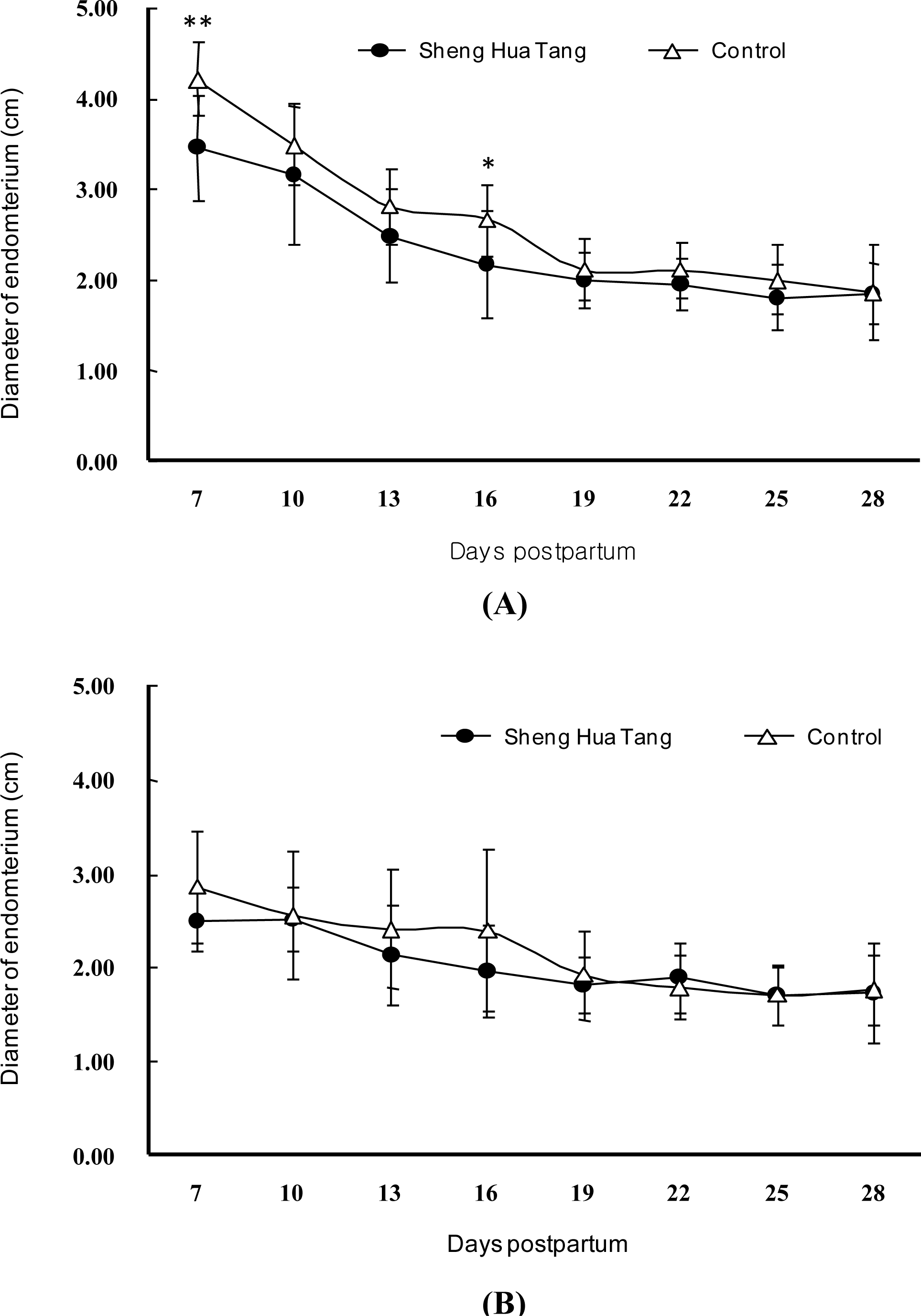
Effects of Sheng Hua Tang treatment on involutionary progress curves for endometrial diameter of uterine horn in postpartum dairy cows. Means±SE (n = 10). (A) The endometrial diameter of gravid uterine horn was obviously reduced in the Sheng Hua Tang-receiving group than the control group on the 7th and 16th d postpartum. * Means differ significantly between groups (p<0.05); ** Means differ significantly between groups (p<0.01); (B) The endometrial diameter of nongravid uterine horn, there were no significant differences in both experimental and control groups.
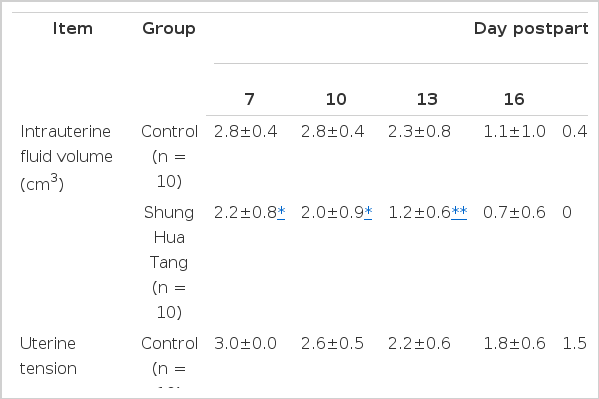
Effects of Sheng Hua Tang treatment on the intrauterine fluid volume and uterine tonicity
The measurements of the intrauterine fluid volumes are shown in Figure 4. The scores for the intrauterine fluid volume in the SHT-treated group were significantly lower than for the control group at 7, 10 (p<0.05), and 13 d postpartum (p<0.01) (Table 2). Fluid in the lumen of the uterine horns was decreased and had a score of 0 on d 25 and 19 postpartum in the control and experimental groups, respectively. The recovery of uterine tonicity in the SHT-treated group was earlier than in the control group. The score for uterine tonicity in the SHT-treated group was significantly lower than in the control group on d 19 (p<0.01) (Table 2). The uteri of all cows receiving SHT had the highest tonicity (score = 1) on d 19 postpartum. However, the uterine tonicity of all control cows was completely recovered on d 28 postpartum.

Ultrasonogram of intrauterine fluid volumes in postpartum cows treated with Sheng Hua Tang. The intrauterine fluid volumes were scored in a 0 to 3 scale: 0 represents no fluid and 1 to 3 represent the increasing fluid volumes in the uterine horns. Indicates a-b-c-d regarding the scope is the endometrium, the arrow for the intrauterine fluid; A: In massive intrauterine fluid, score = 3; B: In quantity intrauterine fluid, score = 2; C: In few intrauterine fluid, score = 1; D: None intrauterine fluid, score = 0.
Effect of Sheng Hua Tang treatment on the progesterone and first ovulation
Ultrasound of the corpus luteum and the concentration of plasma progesterone (≥1.0 ng/mL) were used to detect the first ovulation as an indication for the resumption of ovarian activity. Table 3 shows that ovarian activity resumed in six of the SHT-treated cows (60%) on d 19 postpartum. At the same time, four cows (40%) in the control group were detected to have resumption of ovarian activity. The results showed that the first ovulation was earlier (p = 0.06) in the SHT-treated cows than in the control group.
DISCUSSION
SHT is a well known traditional Chinese medicine therapeutic formula for improving uterine contraction for the discharge of blood, dead tissue and mucus (Bensky et al., 1993). SHT had been shown to improve the recovery of patient with incomplete miscarriage (Cao et al., 2008) or vaginal hemorrhage resulted from abortion procedures (Zhao et al., 2008). In dairy cows, myometrial contractility plays a major role in clearing lochial debris from the uterus after calving (Slama et al., 1991; Hirsbrunner et al., 2002). Normal myoelectrical activity of the uterus is greater during calving and decreases significantly around seven to nine days postpartum (Gajewski and Faundez, 1992; Gajewski et al., 1999). SHT had also been shown to have similar effects in animal models. These effects include increased myoelectric activity of uterine muscles in rabbits (Hong et al., 2003), and the induction of uterus contraction in mice (Zhao et al., 2003). The positive effects of SHT on uterine involution and ovarian activity, in postpartum dairy cows, have been demonstrated in this study.
Ultrasonography is a simple method to use for the diagnosis uterine involution in dairy cows. The endometrial diameters and areas of gravid horns as well as the intrauterine fluid volumes were significantly reduced on d 7 postpartum in the experimental group (p<0.01) compared to the control group. These findings indicate that SHT promoted myometrial contractility after calving. Complete uterine involution was detected in all SHT-treated and control cows two to four weeks postpartum, as reported in previous studies (Hussain and Daniel, 1991; Kindahl et al., 1999). In 2011 (Ho et al.) showed that when SHT was used in treating postpartum women, it can increase the contractile activity of the uterus, and also aid the uterus in returning to its anteverted position. However, there was no significant difference observed in the endometrial areas and diameters of the non-gravid uteri of the experimental and control cows. These findings suggest that the non-gravid horns did not completely expand and were less injured during delivery in spite of bacterial contamination.
Previous studies reported that approximately 80 to 100% of cows may be found to have bacterial contamination in the uterus during the first two weeks postpartum (Paisley et al., 1986; Dohmen et al., 1995; Sheldon et al., 2004). Due to the promotion of uterine involution by SHT and early discharge of uterine fluid there was no pus discharged from the vagina. On the other hand, uterine tonicity is usually reduced due to damage to the uterus during calving. The uterine tonicity recovered on d 19 postpartum (p<0.01) in the SHT-treated group, earlier than the control group in this study.
The resumption of ovarian activity is crucial for subsequent fertility (Darwash et al., 1997; Roch et al., 2000). Ovulation within three weeks postpartum might be an early index for recovery of normal ovarian function and subsequent reproductive performance in high producing dairy cows (Kawahima et al., 2006). The first ovulation occurred in four cows treated with SHT on d 16 postpartum; this was earlier than in the control cows. There were more experimental cows (70%) with their first ovulation than control cows (50%) before d 28 postpartum. The results suggest that SHT aids in the resumption of ovarian activity. The cows were randomly selected and fed with the same diet at the same farm. All cows in the experimental and control groups, without periparturient insults and uterine infection, were examined clinically by ultrasound and during the first four weeks of postpartum. The occurrences of other disorders, such as milk fever or ketosis, were not observed for all the cows during the period of the experiment. Thus, the effects of periparturient disease and diet can be eliminated. The results of this study indicate that uterine involution and ovarian resumption are hastened by treatment with SHT. Taken together, the results of this study show that the Chinese herbal medicine SHT promotes uterine involution and ovarian activity in postpartum dairy cows.
The positive effects of SHT in postpartum animals may be explained by the known pharmacological actions of the five different ingredients used in preparing SHT. These ingredients include angelicae polysacchride, ligustazing, semen persicae, glycyrrhizae radix, and ginger constituents. Angelicae polysacchride, a polysacchride isolated from Radix Angelicae Sinensis, may stimulate the microenvironment within the body, to accelerate the synthesis and secretion of hematopoietic regulation factors. These effects of angelicae polysacchride had been studied and confirmed at the level of gene and protein expression (Wang et al., 1996; Wang et al., 2004; Li et al., 2005). Ligustrazing, one of the active components of the Ligustici Rhizoma, functions by promoting blood circulation and removing blood stasis (Liu et al., 2005). Semen Persicae was shown to possess anti-thrombotic effects (Wang et al., 2002). Glycyrrhizae Radix acts as a potent antispasmodic through the inhibition of phosphodiesterase 3 (Sato et al., 2006). And finally, ginger constituents, extracted from Zingiberis Rhizoma, may reduce inflammation by interfering with the inflammatory cascade (Chrubasik et al., 2005). Although SHT can trigger beneficial pharmacological effects in postpartum dairy cows, further investigations are needed to study the actual molecular mechanisms that triggered these effects.
Acknowledgements
The authors would like to thank the Council of Agriculture of Taiwan for supporting the research. This work was supported by the Council of Agriculture, Executive Yuan, Taiwan, ROC.