 |
 |
Abstract
Eight multiparous Holstein cows (569±47 kg of BW; 84±17 DIM) were used to evaluate the effects of different levels of coarsely ground wheat (CGW) as replacements for ground corn (GC) in diets on feed intake and digestion, ruminal fermentation, lactation performance, and plasma metabolites profiles in dairy cows. The cows were settled in a replicated 4×4 Latin square design with 3-wk treatment periods; four cows in one of the replicates were fitted with rumen cannulas. The four diets contained 0, 9.6, 19.2, and 28.8% CGW and 27.9, 19.2, 9.6, and 0% GC on dry matter (DM) basis, respectively. Increasing dietary levels of CGW, daily DM intake tended to increase quadratically (p = 0.07); however, apparent digestibility of neutral detergent fiber (NDF) and acid detergent fiber (ADF) were significantly decreased (p<0.01) in cows fed the 28.8% CGW diets. Ruminal pH remained in the normal physiological range for all dietary treatments at all times, except for the 28.8% CGW diets at 6 h after feeding; moreover, increasing dietary levels of CGW, the daily mean ruminal pH decreased linearly (p = 0.01). Increasing the dietary levels of CGW resulted in a linear increase in ruminal propionate (p<0.01) and ammonia nitrogen (NH3-N) (p = 0.06) concentration, while ruminal acetate: propionate decreased linearly (p = 0.03) in cows fed the 28.8% CGW diets. Milk production was not affected by diets; however, percentage and yield of milk fat decreased linearly (p = 0.02) when the level of CGW was increased. With increasing levels of dietary CGW, concentrations of plasma beta-hydroxybutyric acid (BHBA) (p = 0.07) and cholesterol (p<0.01) decreased linearly, whereas plasma glucose (p = 0.08), insulin (p = 0.02) and urea nitrogen (p = 0.02) increased linearly at 6 h after the morning feeding. Our results indicate that CGW is a suitable substitute for GC in the diets of dairy cows and that it may be included up to a level of 19.2% of DM without adverse effects on feed intake and digestion, ruminal fermentation, lactation performance, and plasma metabolites if the cows are fed fiber-sufficient diets.
A rapid growth in the the corn ethanol industry has created a need for alternatives to corn grains for lactating dairy cows. Traditionally, wheat is primarily used for human food due to its high nutritive value. However, there is always a great deal of poor-quality (non-moldy) wheat not fit for human consumption or surplus wheat available in some areas, which can be used as an alternative feed for lactating dairy cows if the other feed grains are insufficient or if the price is too high.
The data show that both wheat and corn contain large amounts of starch (77% vs 72%, respectively) (Huntington, 1997), but they differ in the proportion of starch digested in the rumen. Less than 10% of wheat starch remains undegraded in the rumen, but 40% of corn starch remains undegraded (Orskov, 1986). Increasing ruminally available energy content in the diets of dairy cows has the potential for improved efficiency of nutrient utilization in the rumen and increased microbial protein yield, which would enhance the milk production through increased metabolizable nutrient supply (Gozho and Mutsvangwa, 2008). However, highly fermentable diets are rapidly converted to organic acids within the rumen and they readily dissociate to decrease pH. This process can increase the risk of subacute and acute acidosis, inhibit fiber digestion by bacteria (Plaizier et al., 2001) and it is associated with perturbations of the plasma profiles (Ametaj et al., 2009).
Although there have been many studies on the effect of wheat (e.g., cracked, NaOH treatment, steam-rolled) on dairy cows performance (De Campeneere et al., 2006), there is little published information on the potential use of coarsely ground wheat (CGW) as a primary ingredient in rations for lactating dairy cows. Therefore, the objective of the present experiment was to investigate the effect of a linear increase in the level of CGW as a substitute for ground corn (GC) in diets on ruminal fermentation characteristics, feed intake and digestion, lactation performance and plasma metabolites profiles in lactation Holstein cows.
Animal care and procedures were approved and conducted under established standards of the College of Animal Science and Technology, China Agricultural University. Eight healthy, multiparous, lactating Holstein cows (569±47 kg of BW; 84±17 DIM; mean±SD), four of which were fitted with ruminal cannulas (10 id; Bar Diamond, Parma, ID), were used in a replicated 4×4 Latin square design with 21-d periods and four dietary treatments. The fistulated cows formed one replicate within each treatment to monitor ruminal fermentation.
The experiment was conducted at the experimental dairy farm of the State Key Laboratory of Animal Nutrition (Beijing, China). The cows were housed in individual tie-stalls bedded with rubber mattresses and free access to drinking water throughout the trial.
The diets were fed as total mixed ration (TMR) (CAU-mixer wagon model JZC-200, Beijing, China) and formulated with different levels of CGW and GC; the ratio of concentrate to forage was kept constant and equal to 53:47 on DM basis. The four hard red winter wheat levels in the four TMRs were 0, 9.6, 19.2, and 28.8% DM, and the four yellow dent corn levels were 27.9, 19.2, 9.6, and 0% DM. The levels of replacement were chosen in this study, due to loose feces being observed in the high CGW diets (30%, DM basis) in the original design (i.e., 45:55 forage to concentrate) when the first period experiment was conducted. Consequently, the concentrate feeding levels were reduced (i.e., 47:53 forage to concentrate) in all diets. The wheat and corn were ground in a multi-cavity hammer mill (Model No. Z6037; Shuangyi, Tangshan, China) and passed through 3.0-mm and 3.5-mm screens, respectively. Geometric mean particle sizes of the ground wheat and corn were 1,139 μm and 666 μm, respectively (ASAE, 1983). The four diets (Table 1) were formulated to meet or exceed the NRC (2001) guidelines for 600 kg multiparous Holstein dairy cows producing 28 kg of milk/d with 4.0% fat. All diets were formulated to be isoenergetic and isonitrogenous, by adjusting the levels of soybean meal and ruminally inert fats. All ingredients were purchased and prepared once before the start of the experiment. The forage component of the diets was a mixture of corn silage, chopped alfalfa hay, and Chinese wild rye. Moisture content of the silage was determined weekly and used to make ration adjustments. During each data collection period, particle size distribution of TMR was determined using a Penn State Particle Separator (PSPS) as described by Lammers et al. (1996). Percentages (as-fed basis) of the TMR retained on the 19.0-, 8.0-, and 1.18-mm screens, and the bottom pan of the PSPS were 32.5, 19.6, 29.0, and 18.9%, respectively, and numerically similar across diets.
Each experimental period consisted of a 14-d period of adaptation to the diets, followed by 7-d (d 15 to 21) for sample collection and other measurements. The first 3 d of each period were used to adjust the dairy cows gradually to their new diets. The cows were fed twice daily, in equal amounts, at 0700 and 1900 h. The diets were fed ad libitum to allow for at least 5 to 10% orts on an as-fed basis.
During the last 7 days of each period, diet and ort samples of individual cows were harvested daily to calculate feed intake. From d 18 to 20, fecal grab samples (300 to 500 g fresh basis) were collected on 12 occasions, at 0400, 0900, 1400, 1900, 0500, 1000, 1500, 2000, 0600, 1100, 1700, and 2200. The daily diets, orts, and fecal matter were pooled by dietary treatment, period, and cows, and stored at −20°C until analysis. After the experiment, all samples were dried at 65°C in a forced-air oven (Model 2000; Experimental Mill, Beijing, China) for 48 h to a constant weight, ground through a 1-mm screen using a Wiley mill (standard model 4; Arthur H. Thomas Co., Philadelphia, PA, USA), and analyzed for DM, ADF (method 973.18c; AOAC, 1990), and starch (Bal et al., 2000). The NDF was measured by the method of Van Soest et al. (1991) with heat-stable α-amylase (A-3306; Sigma Chemical Co., St. Louis, MO, USA), and sodium sulfite and ash concentration was corrected for the Ankom 200 fiber analyzer (Ankom Technology, Fairport, NY, USA). The CP was determined by the micro-Kjeldahl method (method 4.2.08; AOAC, 1990). The ether extract (method 920.85; AOAC, 1990), calcium and phosphorus (method 945.46; AOAC, 1990) were also analyzed.
The acid-insoluble ash (AIA) was used as an intrinsic digestibility marker to estimate nutrient digestibility in the total tract. The AIA in the diets and feces were analyzed according to Van Keulen and Young (1977), using the equation described by Zhong et al. (2008) to calculate the apparent digestibility of a nutrient in the gastrointestinal tract. The equation is as follows: D = (1-(Ad×Nf)/(Af×Nd)) ×100,where Ad (g/kg) and Af (g/kg) represent the AIA in the diets and feces, respectively, and Nd (g/kg) and Nf (g/kg) represent the nutrient in the diets and feces, respectively.
Ruminal samples were collected for pH, volatile fatty acids (VFA) and NH3-N analysis. Ruminal fluid (100 ml) was sampled at 0700 (before the meal), 1000, 1300, 1600 and 1900 h on d 16 and 17, collected manually from the anterior dorsal, anterior ventral, medial ventral, posterior dorsal, and posterior ventral locations within the rumen, composited by cows. Samples were filtered through four layers of cheesecloth. At each sampling time, the pH was measured immediately after collection using a hand-held pH electrode (Model pH B-4; Shanghai Chemical, Shanghai, China). The NH3-N concentration of ruminal samples was determined using a phenol-hypochlorite assay (Broderick and Kang, 1980). For VFA analysis, a 20-ml filtered sample was put into a plastic bottle with 3 ml of 25% metaphosphoric acid and 3 ml of 0.6% 2-ethyl butyric acid (internal standard), and stored at −20°C. The VFA concentration and profile were determined as described by Cao et al. (2008), using gas chromatography (7980; Agilent Technologies Inc, Santa Clara, CA) equipped with a 30 m HP-INNOWax 19091N-213 (Agilent) capillary column (0.32 mm id and 0.50 mm film thickness).
he cows were milked twice daily, at 0630 and 1830 h. Milk weights were recorded during the 7-d data collection period, and milk samples were taken from d18 to 20 of each experimental period. Milk samples (protein, fat, lactose, and solids nonfat content (SNF)) were analyzed by the Beijing Dairy Cattle Center with a near-infrared reflectance spectroscopy analyzer (MilkoScan 605; Foss Electric, Hillerød, Denmark). Milk production was converted to 3.5% fat-corrected milk (FCM) yield as (0.434×kg of milk) +(16.216×kg of milk fat). The energy corrected milk (ECM) yield was calculated with the equation ECM = 0.327×milk (kg)+12.95×fat (kg)+7.20×protein (kg), on the basis of individual cows (Tyrrell and Reid, 1965).
On d 21 of each experimental period, 10 ml of blood samples were collected, via tail venipuncture at 6 h after the morning feeding, into vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA) containing sodium heparin anticoagulant. Plasma was collected after centrifugation at 3,000×g for 10 min, separated into several aliquots, frozen at −20°C, and later analyzed for determination of glucose, insulin, nonesterified fatty acid (NEFA), BHBA, triglycerides, cholesterol and urea nitrogen. All plasma-related measurements were analyzed in duplicate. The levels of glucose, triglycerides, cholesterol and urea in plasma were analyzed using a clinical auto-analyzer (Cobas Integra, C701; Hoffmann-La Roche Ltd, Basel, Switzerland). The glucose and urea nitrogen (PUN) concentrations were determined using the GOD/PAP and Urease-GLDH test kit (Merit Choice Bioengineering Co., Ltd, Beijing, China). The triglycerides and total cholesterol concentrations were determined following the kit instructions (Shensuo Unf Medical Diagnostic Article Co., Ltd, Shanghai, China), using the enzymatic method. The NEFA and BHBA concentrations in plasma were analyzed with a Hitachi 7600 automated biochemistry analyzer (Hitachi Co., Tokyo, Japan). The NEFA concentrations were determined using a commercially available kit (Sekisui Medical Co., Ltd, Tokyo, Japan). BHBA dehydrogenase was used for quantifying the plasma concentration of BHBA using a commercially available kit (Jingyuan Medical Co., Ltd, Shanghai, China). The plasma insulin levels were determined using an insulin radioimmunoassay kit (Beijing North Institute of Biological Technology, Beijing, China) with a radioimmunoassay system (xh6080; Xi’an Nuclear Instrument Factory, Xi’an, China) according to the manufacturer’s instructions.
Data on DMI, digestibility, lactation performance and plasma metabolite profile were analyzed statistically according to a replicated Latin square design using the GLM procedure of the SAS Institute (2002), using the following model: Yijkl = μ+Pi+Cj(l)+Tk+Sl+STlk+Eijkl, where Yijkl = dependent variable; μ = overall mean; Pi = effect of period i; Cj(l) = effect of cow j within square l; Tk = effect of treatment k; Sl = square effect (l = 1 or 2); STlk = interaction between square l and treatment k; and Eijkl = the random residual error.
Rumen fermentation data, which had repeated measures over time, was analyzed using the following model: Yijkm = μ+Pi+Cj+Tk+Hm+HTmk+Eijkm, where Yijkm = dependent variable; μ = overall mean; Pi = effect of period i; Cj = effect of cow j; Tk = effect of treatment k; Hm = effect of hours post-feeding analyzed as repeated measures; HTmk = interaction between hour m and treatment k; and Eijkm = random residual error.
The above data were compared by Tukey’s range test. Orthogonal polynomial contrasts were used to examine the responses (linear and quadratic) to increasing the substitution level of CGW for GC in the diets. In orthogonal polynomial analysis, coefficients were corrected due to unequal spacing of treatment.
In the current study, DM intake (DMI) was not significantly affected by the levels of CGW in the diet; however, increasing levels of CGW resulted in a quadratic pattern (p = 0.07) in daily DMI, which was maximized when cows were fed the 9.6 and 19.2% CGW diets, and then declined when CGW was included at 28.8% of diet DM (Table 4). Apparent digestibility of CP was not altered in response to CGW feeding (Table 2); however, linear decreases were observed in apparent DM (p = 0.02), NDF (p<0.01), and ADF (p<0.01) digestibility with increasing levels of CGW in the diet. Moreover, digestibility of NDF and ADF decreased significantly (p<0.01) in cows fed 28.8% CGW diets. Apparent digestibility of starch responded quadratically (p = 0.03) to the replacement of GC with CGW.
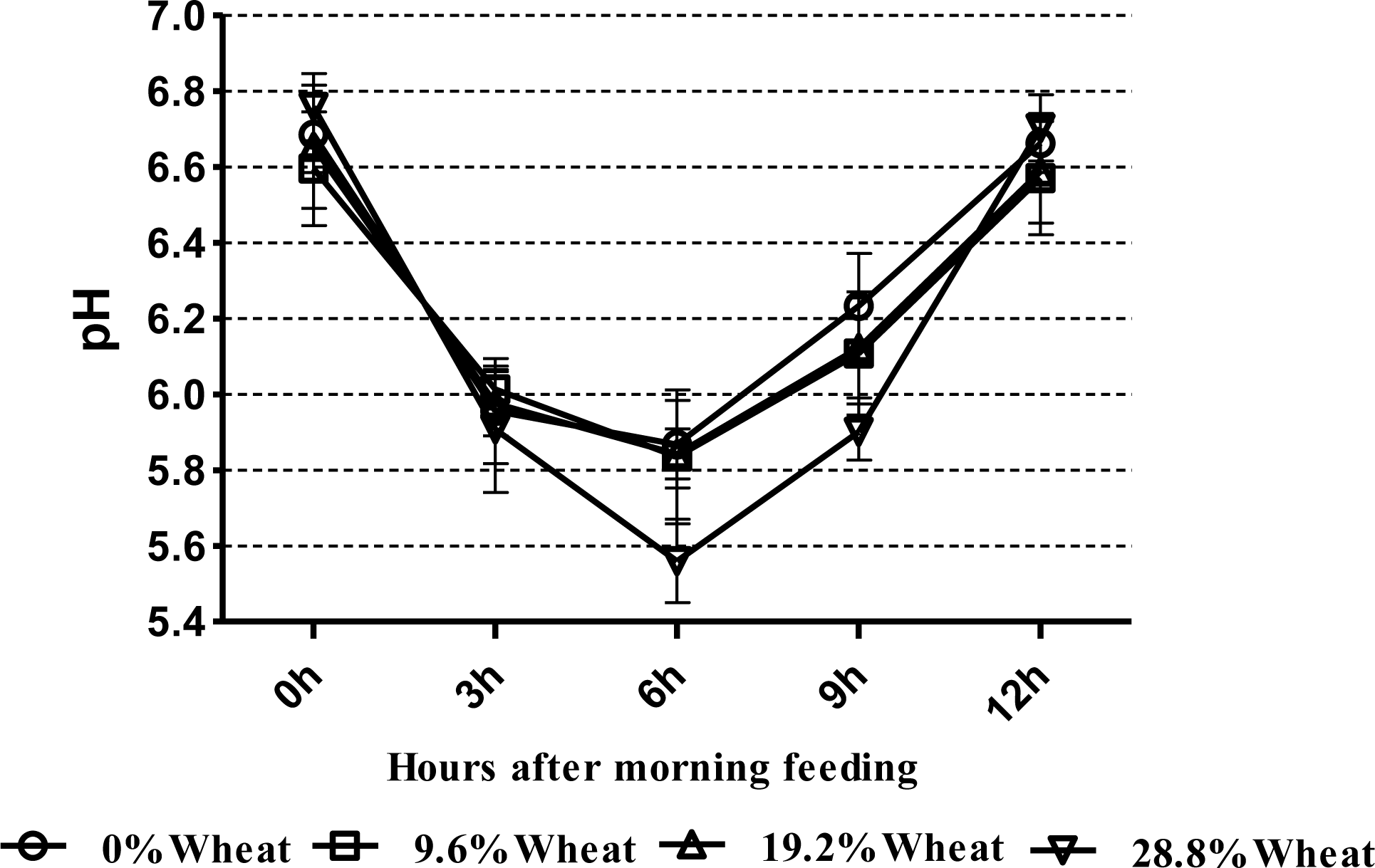
Ruminal pH remained in the normal physiological range for all dietary treatments at all times, except for the 28.8% CGW diets at 6 h after feeding (Figure 1); moreover, with increasing levels of CGW in the diet, the daily mean ruminal pH decreased linearly (p = 0.01) (Table 3). Concentrations of ruminal NH3-N tended to increase linearly (p = 0.06) with increasing levels of dietary CGW. Neither concentration nor percentage of acetate, butyrate, valerate, isobutyrate and isovalerate were affected by the diets; however, ruminal propionate concentration increased linearly (p<0.01) when the level of dietary CGW was increased, while the acetate to propionate ratio decreased linearly (p = 0.03) when CGW was included at 28.8% of diet DM. The percentage of propionate increased linearly (p<0.01) in cows fed the 28.8% CGW diets. Total VFA concentration increased quadratically (p = 0.04) with increasing levels of dietary CGW.
Milk yield was not affected by diet, and it averaged 27.1 kg/d; however, milk yield was numerically lower for the 28.8% CGW diets (Table 4). The percentage and yield of milk protein, lactose, and SNF were not different across diets; however, increasing levels of dietary CGW resulted in a linear decrease in milk fat percentage (p = 0.02), with a tendency for a linear decrease in milk fat yield (p = 0.02), 3.5% FCM yield (p = 0.05), and ECM yield (p = 0.06). Feed efficiency expressed as milk/kg of DMI was not affected by diet; however, 3.5% FCM/DMI decreased linearly (p = 0.04) and quadratically (p = 0.01) when the level of CGW was increased. Nitrogen efficiency was not affected by diet.
The concentrations of plasma BHBA (p = 0.07) tended to decrease linearly, whereas plasma glucose (p = 0.08), insulin (p = 0.02) and urea nitrogen (p = 0.02) tended to increase linearly, with increasing dietary CGW levels; plasma NEFA was not affected by diet (Table 5). Plasma cholesterol concentrations decreased linearly (p<0.01) with increasing dietary CGW levels, and decreased significantly (p<0.01) in cows fed the 28.8% CGW diets. Plasma triglyceride concentration tended to respond quadratically (p = 0.08) to the replacement of GC with CGW.
Wheat has generally been considered a risk to reduce feed intake for its higher ruminally available starch (NRC, 2001); however, DMI was not significantly affected by replacing GC with CGW in the current study. Recent research indicates that dairy cows fed 20% dietary DM steam-rolled wheat, in place of steam-rolled barley, had similar feed intakes (Doepel et al., 2009). Faldet et al. (1989) also showed that the concentrate mixtures with 60% wheat had no influence on the DMI of dairy cows. In contrast, increasing levels of wheat in the diets decreased DMI in other studies (Gozho and Mutsvangwa, 2008; Lechartier and Peyraud, 2010). In the current study, DMI tended to increase quadratically with increasing levels of dietary CGW; this may be a reflection of the CGW having higher palatability than GC for dairy cows. Nikkhah et al. (2010) indicated that 18% dietary DM ground wheat could feasibly be fed to periparturient cows to improve DMI and energy balances.
In the present study, apparent digestibility of DM, NDF, and ADF were reduced in cows fed the 28.8% CGW diets. This result may reflect the negative associated effects of fermentable carbohydrates and fiber degradability in the rumen (Firkins, 1997). Ruminal pH decreased to 5.56 at 6 h after feeding when cows were fed the 28.8% CGW diets in this trial (Figure 1), resulting in the lower digestibility of the NDF (Leddin et al., 2009). An in vitro study by Stensig et al. (1998) showed that with a higher proportion of wheat starch in the diet, the rate of digestion and passage of NDF decreased. However, Gozho and Mutsvangwa (2008) did not observe that total tract digestibility of DM, OM, and NDF were affected by the dietary source of carbohydrate, but total tract ADF digestibility tended to be lower in cows fed the wheat-based (32.8%, DM basis) TMR compared with those fed the corn-based (28.7%, DM basis) TMR. Martin et al. (1999) reported that ruminal NDF digestibility and most fibrolytic activities of the solid-associated microorganisms were lower in beef steers fed wheat-based diets than in those fed corn-based diets. In dairy cows, the main site of cereal grain starch digestion is the rumen, and wheat exhibits faster and more extensive ruminal starch degradation compared with the corn (Huntington, 1997). It was expected that total tract starch digestibility would be greater in cows fed the CGW. However, in this trial, the lower digestibility of starch occurred in the 19.2% CGW diets. This was unexpected and is difficult to explain. Apparent total tract digestibility of CP was not different among the treatments, and is in agreement with other studies (Doepel et al., 2009; Gozho and Mutsvangwa, 2008).
In our study, ruminal pH remained in the normal physiological range for all dietary treatments, except for the 28.8% CGW diets at 6 h after feeding. Lower ruminal pH might increase the risk of subacute ruminal acidosis (SARA). It is recognized that SARA occurs in repeated periods of lowered pH, below 5.6 to 5.8 (Lechartier and Peyraud, 2011). Our results are similar to findings from the study conducted by Doepel et al. (2009), who determined that 10 or 20% of dietary DM steam-rolled wheat led to lower ruminal pH, but all dietary treatments remained in the normal physiological range. Moreover, Lechartier and Peyraud (2011) showed that increasing wheat (14.6 and 29.7%, DM basis) in the diets increased ruminal pH range and linearly decreased ruminal fibrolytic activity, estimated from the disappearance of soybean hull DM after 24 h of in sacco incubation. An in vitro experiment showed that the acid-producing potential of wheat was higher than that of corn (acidogenicity values, 17.2 vs 12.6, respectively), and high levels of wheat inclusion in the diets increased ruminal lactic acid concentration in accordance with decreased rumen pH (Wadhwa et al., 2001). In contrast, Gozho and Mutsvangwa (2008) have reported similar ruminal pH levels in cows fed corn or wheat based TMR, which is difficult to explain biologically.
As described above, ruminal NH3-N concentration tended to increase linearly with increasing levels of dietary CGW. The increase in ruminal NH3-N, possibly resulting from a reduction in microbial capture of released NH3 (Kolver et al., 1998), suggests that nitrogen efficiency is lower. This is also reflected in the linear increase in PUN. In contrast, Gozho and Mutsvangwa (2008) reported that ruminal NH3-N concentration was unaffected by dietary treatment (wheat or corn).
In the present research, ruminal propionate concentration increased linearly as the levels of dietary CGW increased, which is similar to the results of previous studies (Doepel et al., 2009; Lechartier and Peyraud, 2011). The variations in propionate concentrations and acetate to propionate ratio indicates a shift in ruminal fermentation pattern consistent with starch fermentability of the diets, and in accordance with the tendency toward a decrease in milk fat percentage or yield in our study. An in vitro study conducted by Russell (1998) showed that propionate production increased due to the ability of starch-degrading bacteria, which prefer to produce propionate. Increased total VFA concentration is an indication for either a higher rate of VFA production or a slower rate of VFA disappearance as levels of CGW increased in the diets. In general, the pH decreases with increasing ruminal VFA concentration after feeding (Dijkstra et al., 2012). However, cows that were fed the 28.8% CGW diets had lower ruminal pH values and total VFA concentration than other diets in this trial, which seems to be contradictory. Allen (1997) concluded that the relationship between ruminal VFA concentration and ruminal pH appears to be weak; the reason being related to large variations among diets in the removal, buffering, and neutralization of acids in the rumen. Furthermore, our results may be linked to the cows own regulation of microbial adaptation and VFA absorption (Krause and Oetzel, 2006), when fed diets containing high amounts of rapidly fermentable carbohydrates.
In our study, milk yield was similar across dietary treatments, but numerically lower for the 28.8% CGW diet. In agreement with our findings, Doepel et al. (2009) observed no effect of 20% dietary DM steam-rolled wheat on milk yield of dairy cows. Gozho and Mutsvangwa (2008) reported that milk yield was being negatively affected by high levels of wheat which was probably caused by the diurnal pattern of ruminal pH. Cows fed the 0, 9.6, and 19.2% CGW diets had numerically greater milk 3.5% FCM and ECM yield values compared with those fed the 28.8% CGW diets, which could be due to the variation of milk yield and milk fat percentage in our study.
Milk fat percentage and yield decreased in cows fed the 28.8% CGW diets, indicating that feeding large amounts of CGW to dairy cows can negatively affect milk fat concentration, a result similar to that of previous research studies (Gozho and Mutsvangwa, 2008; Lechartier and Peyraud, 2011). This result could be attributed to the increased ruminal propionate production in our study, which may limit the flux of milk fat precursors towards the mammary gland (Doreau et al., 1999). Moreover, too much rapidly fermented starch had a significant impact on ruminal pH, biohydrogenation, and bacterial community composition, which is associated with milk fat depression (Weimer et al., 2010).
Feed efficiency decreased linearly with the increase in dietary CGW levels, indicating that a large amount of CGW could depress energy utilization efficiency. This result might be caused by the higher levels of rapidly fermented starch tending to exaggerate diurnal patterns of ruminal pH and resulting in alterations of ruminal functions (Zebeli et al., 2011).
The observations obtained in the present study showed that plasma BHBA and cholesterol concentration were lower in the high proportion of CGW diets. Our results are similar to those previously reported by Ametaj et al. (2009). They demonstrated that feeding increasing proportions of barley grain was associated with lower concentrations of plasma BHBA and cholesterol in cows. Plasma BHBA comes from oxidation of NEFA in liver hepatocytes (Roche et al., 2008) or the butyrate (Andersson and Lundstroem, 1985), and lower plasma BHBA concentration is probably due to the variation in its sources. The lower cholesterol is related to changes in plasma amino acids, severity of acute phase response, and metabolic disorder, as reported by Chiarla et al. (2004). Zebeli et al. (2011) demonstrated inverse relationships between rumen endotoxin and plasma cholesterol or BHBA concentrations, which are related to the systemic inflammatory response triggered by translocation of an endotoxin into the peripheral circulation (Khafipour et al., 2009).
Plasma glucose concentrations tended to increase with increasing levels of CGW in the diets. This result might be related to a greater availability of starch from CGW, which provided more glucogenic precursors (propionate) than starch from GC. Van Knegsel et al. (2007) indicated that feeding diets containing large amounts of fermentable carbohydrates is associated with greater plasma glucose concentrations. Propionate and, to a lesser extent, glucose, are insulin secretagogues, and insulin is reported to inhibit hepatic gluconeogenesis (Brockman, 1985). Plasma insulin increased in cows fed high CGW diets in the present research, which indicates an increase in propionate production. The higher plasma glucose and insulin concentrations are also associated with the lower milk fat percentage in this trial. These findings might at least partly account for milk-fat depression, even though the glucogenic theory is still in doubt (Bergen, 2009).
Concentrations of PUN increased in cows fed high CGW diet, which indicates that a great amount of NH3 was released in the rumen after feeding, exceeding the capacity of microbes to utilize it. It is more likely that less NH3 was incorporated into microbial protein due to lower NDF digestion in this study. This led to a large portion of NH3 being absorbed across the rumen wall for urea synthesis in the liver, as well as its subsequent increased concentration in systemic circulation (Burgos et al., 2007). In the present research, plasma triglyceride concentration tended to increase quadratically as dietary CGW level increased in diets, a finding in agreement with the observations of Bradford and Allen (2004), who indicated that feeding easily degradable starch diets increased plasma triglyceride concentrations. The lower plasma triglyceride in cows fed the 28.8% CGW diet was probably due to decreased lipolysis and ruminal biohydrogenation at low rumen pH (Jenkins, 1993), thereby reducing triglyceride concentrations in peripheral blood circulation.
Our study revealed that CGW is a suitable substitute for GC in diets of lactating dairy cows when fed up to 19.2% of dietary DM. A practical concern with feeding CGW above 19.2% of dietary DM is that it could negatively affect nutrient digestibility, ruminal pH, and milk fat concentration, and potentially alter the plasma metabolites.
ACKNOWLEDGMENTS
The study was supported by the funds from the National Key Basic Research Program of China (Project No. 2011CB100801) and National Science Foundation of China (30901030).
Table 1.
Ingredient and nutrient composition of experimental diets
| Item |
Dietary treatment (% CGW)
|
|||
|---|---|---|---|---|
| 0 | 9.6 | 19.2 | 28.8 | |
| Ingredient/diets (% of DM) | ||||
| Corn silage | 27.0 | 27.0 | 27.0 | 27.0 |
| Alfalfa hay | 14.0 | 14.0 | 14.0 | 14.0 |
| Chinese wildrye | 6.0 | 6.0 | 6.0 | 6.0 |
| Corn(ground) | 27.9 | 19.2 | 9.6 | 0 |
| Wheat(coarsely ground) | 0 | 9.6 | 19.2 | 28.8 |
| Soybean meal | 10.4 | 9.1 | 7.2 | 5.8 |
| Cottonseed meal | 3.4 | 3.4 | 3.4 | 3.4 |
| DDGS1 | 4.3 | 4.3 | 4.3 | 4.3 |
| Wheat bran | 0 | 0.3 | 2.0 | 3.2 |
| Whole cottonseed | 4.0 | 4.0 | 4.0 | 4.0 |
| Ruminally inert fat2 | 0.1 | 0.3 | 0.5 | 0.7 |
| Mineral-vitamin premix3 | 0.5 | 0.5 | 0.5 | 0.5 |
| Dicalcium phosphate | 0.6 | 0.5 | 0.4 | 0.3 |
| Limestone | 0.8 | 0.8 | 0.8 | 0.8 |
| Sodium bicarbonate | 0.5 | 0.5 | 0.5 | 0.5 |
| Magnesium oxide | 0.2 | 0.2 | 0.2 | 0.2 |
| Salt | 0.4 | 0.4 | 0.4 | 0.4 |
| Chemical composition (% of DM) | ||||
| CP | 15.9 | 16.1 | 16.2 | 16.4 |
| NEL4, Mcal/kg | 1.59 | 1.59 | 1.59 | 1.59 |
| NDF | 35.2 | 35.8 | 36.8 | 37.4 |
| ADF | 20.0 | 20.9 | 20.9 | 21.4 |
| FNDF5 | 30.2 | 30.2 | 30.2 | 30.2 |
| Starch | 31.7 | 31.6 | 31.4 | 31.2 |
| Ether extract | 3.4 | 3.4 | 3.4 | 3.4 |
| Ca | 0.8 | 0.8 | 0.8 | 0.8 |
| Total P | 0.4 | 0.4 | 0.4 | 0.4 |
3 Contained (/kg of premix; DM basis):1,000,000 IU vitamin A; 65,000 IU vitamin D; 5,000 IU vitamin E; 2,000 mg Fe; 2,550 mg Mn; 5,500 mg Zn; 1,750 mg Cu; 70 mg I; 40 mg Co; 75 mg Se.
4 Calculated using NEL values of feedstuffs from NRC (2001).
Table 2.
Effect of replacing dietary GC with CGW on total tract nutrient digestibility in dairy cows (%)
| Item | Dietary treatment (% CGW) | SEM1 | p value | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 0 | 9.6 | 19.2 | 28.8 | Linear | Quadratic | ||
| DM | 68.8 | 67.8 | 67.1 | 66.3 | 0.56 | 0.02 | 0.54 |
| NDF | 53.5a | 52.7a | 49.1ab | 46.4b | 0.86 | <0.01 | 0.85 |
| ADF | 52.4a | 52.3a | 48.8ab | 45.6b | 0.79 | <0.01 | 0.77 |
| CP | 71.4 | 71.4 | 71.2 | 71.0 | 0.50 | 0.69 | 0.95 |
| Starch | 88.4 | 87.6 | 86.3 | 87.8 | 0.53 | 0.47 | 0.03 |
Table 3.
Effect of replacing dietary GC with CGW on pH and concentration of metabolites in the rumen1
| Item | Dietary treatment (% CGW) | SEM2 | p value | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 0 | 9.6 | 19.2 | 28.8 | Linear | Quadratic | ||
| Ruminal pH | 6.28 | 6.22 | 6.23 | 6.16 | 0.05 | 0.01 | 0.98 |
| Ruminal NH3-N (mg/100 ml) | 11.97 | 12.02 | 12.36 | 13.08 | 0.49 | 0.06 | 0.66 |
| VFA concentration (mmol/L) | |||||||
| Acetate | 64.82 | 66.79 | 67.05 | 63.45 | 1.44 | 0.18 | 0.15 |
| Propionate | 22.94b | 23.30b | 23.65ab | 25.29a | 0.87 | <0.01 | 0.32 |
| Butyrate | 8.52 | 9.03 | 9.31 | 8.07 | 0.29 | 0.47 | 0.18 |
| Isobutyrate | 0.83 | 0.85 | 0.87 | 0.77 | 0.02 | 0.42 | 0.36 |
| Valerate | 1.23 | 1.33 | 1.44 | 1.36 | 0.05 | 0.31 | 0.22 |
| Isovalerate | 1.26 | 1.33 | 1.20 | 1.07 | 0.06 | 0.09 | 0.50 |
| Total VFA | 99.61 | 102.64 | 103.51 | 98.89 | 2.14 | 0.51 | 0.04 |
| VFA (mmol/100 mmol total) | |||||||
| Acetate | 65.19 | 65.16 | 64.85 | 64.30 | 0.58 | 0.56 | 0.91 |
| Propionate | 22.87b | 22.54b | 22.69b | 25.39a | 0.74 | <0.01 | 0.03 |
| Butyrate | 8.61 | 8.86 | 9.06 | 8.20 | 0.32 | 0.60 | 0.41 |
| Acetate:propionate ratio | 2.95 | 3.00 | 2.97 | 2.68 | 0.10 | 0.03 | 0.14 |
Table 4.
Effect of replacing dietary GC with CGW on DMI, milk yield and compositions in dairy cows
| Item | Dietary treatment (% CGW) | SEM1 | p value | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 0 | 9.6 | 19.2 | 28.8 | Linear | Quadratic | ||
| DMI (kg/d) | 19.14 | 20.09 | 20.02 | 19.73 | 0.19 | 0.40 | 0.07 |
| Milk (kg/d) | 27.16 | 27.05 | 27.10 | 26.45 | 0.49 | 0.28 | 0.66 |
| 3.5% FCM (kg/d) | 27.72 | 27.68 | 27.75 | 26.45 | 0.49 | 0.05 | 0.27 |
| ECM (kg/d)2 | 28.15 | 28.06 | 28.26 | 26.98 | 0.46 | 0.06 | 0.24 |
| Milk fat (%) | 3.64 | 3.65 | 3.65 | 3.50 | 0.05 | 0.02 | 0.15 |
| Milk protein (%) | 3.34 | 3.31 | 3.36 | 3.33 | 0.03 | 0.98 | 0.81 |
| Milk lactose (%) | 5.04 | 5.07 | 4.99 | 5.04 | 0.03 | 0.84 | 0.57 |
| SNF (%) | 9.09 | 9.09 | 9.13 | 9.10 | 0.04 | 0.77 | 0.69 |
| Milk fat (kg/d) | 0.99 | 0.99 | 0.99 | 0.93 | 0.02 | 0.02 | 0.94 |
| Milk protein (kg/d) | 0.90 | 0.89 | 0.91 | 0.88 | 0.01 | 0.30 | 0.29 |
| Milk lactose (kg/d) | 1.37 | 1.38 | 1.35 | 1.34 | 0.03 | 0.34 | 0.50 |
| SNF (kg/d) | 2.46 | 2.46 | 2.48 | 2.41 | 0.04 | 0.36 | 0.53 |
| Milk/DMI | 1.43 | 1.35 | 1.35 | 1.34 | 0.03 | 0.15 | 0.66 |
| 3.5% FCM/DMI | 1.45 | 1.38 | 1.39 | 1.34 | 0.03 | 0.04 | 0.01 |
| Nitrogen efficiency (g/g)3 | 0.27 | 0.26 | 0.26 | 0.26 | 0.01 | 0.15 | 0.57 |
Table 5.
Effect of replacing dietary GC with CGW on plasma metabolites profiles in dairy cows1
| Item | Dietary treatment (% CGW) | SEM2 | p value | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 0 | 9.6 | 19.2 | 28.8 | Linear | Quadratic | ||
| BHBA (mmol/L) | 0.85 | 0.88 | 0.77 | 0.72 | 0.04 | 0.07 | 0.84 |
| Cholesterol (mmol/L) | 3.25a | 3.20a | 3.14a | 2.68b | 0.08 | <0.01 | 0.22 |
| NEFA (μEq/L) | 108.13 | 107.25 | 114.75 | 110.00 | 2.91 | 0.69 | 0.56 |
| Glucose (mmol/L) | 2.91 | 3.04 | 3.05 | 3.21 | 0.08 | 0.08 | 0.97 |
| Insulin (μIU/ml) | 7.09 | 7.40 | 8.02 | 9.76 | 0.45 | 0.02 | 0.65 |
| PUN (mmol/L) | 5.21 | 5.40 | 5.48 | 5.81 | 0.09 | 0.02 | 0.94 |
| Triglyceride (mmol/L) | 0.17 | 0.18 | 0.18 | 0.17 | 0.01 | 0.60 | 0.08 |
REFERENCES
Allen MS. 1997. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J Dairy Sci 80:1447–1462.


Ametaj BN, Emmanuel DG, Zebeli Q, Dunn SM. 2009. Feeding high proportions of barley grain in a total mixed ration perturbs diurnal patterns of plasma metabolites in lactating dairy cows. J Dairy Sci 92:1084–1091.


Andersson L, Lundstroem K. 1985. Effect of feeding silage with high butyric acid content on ketone body formation and milk yield in postparturient dairy cows. Zentralbl. Veterinaermed A 32:15–23.

AOAC. 1990. Official methods of analysis. 15th edAssociation of Official Analytical Chemists; Washington, DC:
ASAE. 1983. Method of determining and expressing fineness of feed materials by sieving. p. 325ASAE Standard S319, Agriculture Engineers Yearbook of Standards. Am. Soc. Agric. Eng.; St. Joseph, MI:
Bal MA, Shaver RD, Jirovec AG, Shinners KJ, Coors JG. 2000. Crop processing and chop length of corn silage: Effects on intake, digestion, and milk production by dairy cows. J Dairy Sci 83:1264–1273.


Bergen WG. 2009. Milk-fat depression and lipid repartitioning in lactating dairy cows. J Nutr 139:826–827.


Bradford BJ, Allen MS. 2004. Milk fat responses to a change in diet fermentability vary by production level in dairy cattle. J Dairy Sci 87:3800–3807.


Brockman RP. 1985. Role of insulin in regulating hepatic gluconeogenesis in sheep. Can J Physiol Pharmacol 63:1460–1464.


Broderick GA, Kang JH. 1980. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci 63:64–75.


Burgos SA, Fadel JG, DePeters EJ. 2007. Prediction of ammonia emission from dairy cattle manure based on milk urea nitrogen: Relation of milk urea nitrogen to urine urea nitrogen excretion. J Dairy Sci 90:5499–5508.


Cao ZJ, Li SL, Xing JJ, Ma M, Wang LL. 2008. Effects of maize grain and lucerne particle size on ruminal fermentation, digestibility and performance of cows in midlactation. J Anim Physiol Anim Nutr 92:157–167.

Chiarla C, Giovannini I, Siegel JH. 2004. The relationship between plasma cholesterol, amino acids and acute phase proteins in sepsis. Amino Acids 27:97–100.


De Campeneere S, De Boever JL, De Brabander DL. 2006. Comparison of rolled, NaOH treated and ensiled wheat grain in dairy cattle diets. Livest Sci 99:267–276.

Dijkstra J, Ellis JL, Kebreab E, Strathe AB, López S, France J, Bannink A. 2012. Ruminal pH regulation and nutritional consequences of low pH. Anim Feed Sci Technol 172:22–33.

Doepel L, Cox A, Hayirli A. 2009. Effects of increasing amounts of dietary wheat on performance and ruminal fermentation of Holstein cows. J Dairy Sci 92:3825–3832.


Doreau M, Chilliard Y, Rulquin H, Demeyer DI. 1999. Manipulation of milk fat in dairy cows. p. 81–109. Recent Advances in Animal Nutrition. Garnsworthy PC, Wiseman J, editorsNottingham Press; Nottingham, UK:
Faldet MA, Nalsen T, Bush LJ, Adams GD. 1989. Utilization of wheat in complete rations for lactating cows. J Dairy Sci 72:1243–1251.


Firkins JL. 1997. Effects of feeding nonforage fiber sources on site of fiber digestion. J Dairy Sci 80:1426–1437.


Gozho GN, Mutsvangwa T. 2008. Influence of carbohydrate source on ruminal fermentation characteristics, performance, and microbial protein synthesis in dairy cows. J Dairy Sci 91:2726–2735.


Huntington GB. 1997. Starch utilization by ruminants: from basics to the bunk. J Anim Sci 75:852–867.


Khafipour E, Krause DO, Plaizier JC. 2009. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J Dairy Sci 92:1060–1070.


Kolver E, Muller LD, Varga GA, Cassidy TJ. 1998. Synchronization of ruminal degradation of supplemental carbohydrate with pasture nitrogen in lactating dairy cows. J Dairy Sci 81:2017–2028.


Krause KM, Oetzel GR. 2006. Understanding and preventing subacute ruminal acidosis in dairy herds: A review. Anim Feed Sci Technol 126:215–236.

Lammers BP, Buckmaster DR, Heinrichs AJ. 1996. A simple method for the analysis of particle sizes of forage and total mixed rations. J Dairy Sci 79:922–928.


Lechartier C, Peyraud JL. 2010. The effects of forage proportion and rapidly degradable dry matter from concentrate on ruminal digestion in dairy cows fed corn silage-based diets with fixed neutral detergent fiber and starch contents. J Dairy Sci 93:666–681.


Lechartier C, Peyraud JL. 2011. The effects of starch and rapidly degradable dry matter from concentrate on ruminal digestion in dairy cows fed corn silage-based diets with fixed forage proportion. J Dairy Sci 94:2440–2454.


Leddin CM, Stockdale CR, Hill J, Heard JW, Doyle PT. 2009. Increasing amounts of crushed wheat fed with pasture hay reduced dietary fiber digestibility in lactating dairy cows. J Dairy Sci 92:2747–2757.


Martin C, Philippeau C, Michalet-Doreau B. 1999. Effect of wheat and corn variety on fiber digestion in beef steers fed high-grain diets. J Anim Sci 77:2269–2278.


National Research Council (NRC). 2001. Nutrient requirements of dairy cattle. 7th rev edNational Academy Press; Washington, DC, USA:
Nikkhah A, Ehsanbakhsh F, Zahmatkesh D, Amanlou H. 2010. Prepartal wheat grain feeding improves energy and calcium status of periparturient Holstein heifers. Animal 5:522–527.

Plaizier JC, Keunen JE, Walton JP, Duffield TF, McBride BW. 2001. Effect of subacute ruminal acidosis on in situ digestion of mixed hay in lactating dairy cows. Can J Anim Sci 81:421–423.

Roche JR, Sheahan AJ, Chagas LM, Boston RC. 2008. Short communication: change in plasma ghrelin in dairy cows following an intravenous glucose challenge. J Dairy Sci 91:1005–1010.


Russell JB. 1998. The importance of pH in the regulation of ruminal acetate to propionate ratio and methane production in vitro. J Dairy Sci 81:3222–3230.


Stensig T, Weisbjerg MR, Hvelplund T. 1998. Digestion and passage kinetics of fibre in dairy cows as affected by the proportion of wheat starch or sucrose in the diet. Acta Agric Scand A Anim Sci 48:129–140.

Van Keulen J, Young BA. 1977. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J Anim Sci 44:282–287.

Van Knegsel A, Van den Brand H, Graat E, Dijkstra J, Jorritsma R, Decuypere E, Tamminga S, Kemp B. 2007. Dietary energy source in dairy cows in early lactation: metabolites and metabolic hormones. J Dairy Sci 90:1477–1485.


Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597.


Wadhwa D, Beck NFG, Borgida LP, Dhanoa MS, Dewhurst RJ. 2001. Development of a simple in vitro assay for estimating net rumen acid load from diet ingredients. J Dairy Sci 84:1109–1117.


Weimer PJ, Stevenson DM, Mertens DR. 2010. Shifts in bacterial community composition in the rumen of lactating dairy cows under milk fat-depressing conditions. J Dairy Sci 93:265–278.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





