Effect of Carbohydrate Source and Cottonseed Meal Level in the Concentrate on Feed Intake, Nutrient Digestibility, Rumen Fermentation and Microbial Protein Synthesis in Swamp Buffaloes
Article information
Abstract
The objective of this study was to investigate the effect of carbohydrate source and cottonseed meal level in the concentrate on feed intake, nutrient digestibility, rumen fermentation and microbial protein synthesis in swamp buffaloes. Four, 4-yr old rumen fistulated swamp buffaloes were randomly assigned to receive four dietary treatments according to a 2×2 factorial arrangement in a 4×4 Latin square design. Factor A was carbohydrate source; cassava chip (CC) and CC+rice bran at a ratio 3:1 (CR3:1), and factor B was level of cottonseed meal (CM); 109 g CP/kg (LCM) and 328 g CP/kg (HCM) in isonitrogenous diets (490 g CP/kg). Buffaloes received urea-treated rice straw ad libitum and supplemented with 5 g concentrate/kg BW. It was found that carbohydrate source did not affect feed intake, nutrient intake, digested nutrients, nutrient digestibility, ammonia nitrogen concentration, fungi and bacterial populations, or microbial protein synthesis (p>0.05). Ruminal pH at 6 h after feeding and the population of protozoa at 4 h after feeding were higher when buffalo were fed with CC than in the CR3:1 treatment (p<0.05). Buffalo fed with HCM had a lower roughage intake, nutrient intake, population of total viable and cellulolytic bacteria and microbial nitrogen supply than the LCM fed group (p<0.05). However, nutrient digestibility, ruminal pH, ammonia concentration, population of protozoa and fungi, and efficiency of microbial protein synthesis were not affected by cottonseed meal levels (p>0.05). Based on this experiment, concentrate with a low level of cottonseed meal could be fed with cassava chips as an energy source in swamp buffalo receiving rice straw.
INTRODUCTION
The considerable increase in feed costs when animal diets are based on imported feedstuffs has necessitated a search for cheaper energy and protein sources on farm to replace expensive feed resources. Cassava (Manihot esculenta) tubers contain high levels of energy (Wanapat, 2003) and have been used as a source of readily fermentable energy in beef cattle (Wanapat and Khampa, 2007), dairy cattle (Khampa et al., 2006) and buffalo (Etman et al., 1993; Wanapat et al., 2009) rations. Moreover, by-products from oilseed factories are amongst the potential protein sources available widely (Wanapat, 2009). This is especially true of cottonseed meal which contains a high proportion of rumen undegradable protein (Grings et al., 1991). These could be beneficial for improvement of animal performance by incorporating with low degraded carbohydrate. Rice bran was combined with cassava chip, as it is slowly degraded carbohydrate in the rumen which could be incorporated with cottonseed meal for improvement of animal production. Our previous studies revealed that using combination of cassava chip and rice bran as carbohydrate sources can improve digestibility, populations of total viable bacteria and proteolytic bacteria (Wanapat et al., 2013). While, using high level of cotton seed meal in the diet, it can further improve total feed intake, milk yield, milk composition and milk income in dairy cows (Wanapat et al., 2012). Moreover, nitrogen balance and volatile fatty acids were remarkably increased when young dairy bulls fed on the concentrate with cottonseed meal (Wanapat et al., 2013). However, the study of carbohydrate source and cottonseed meal level has not been done with swamp buffaloes. Therefore, this study was conducted to investigate the effects of carbohydrate source and level of cottonseed meal in the concentrate on dry matter intake, nutrient digestibility, rumen fermentation, microbial population and microbial protein synthesis in swamp buffaloes fed on a diet based on urea-treated rice straw.
MATERIALS AND METHODS
Animals and feeds
Four, 4-yr old rumen fistulated swamp buffalo bulls with a body weight of 400±15 kg were randomly assigned to receive dietary treatments according to a 2×2 factorial arrangement in a 4×4 Latin square design with 4 dietary treatments. Factor A was carbohydrate source; cassava chip (CC): CC+rice bran in a ratio of 3:1(CR3:1), and Factor B was level of cottonseed meal (CM): 109 g CP/kg (LCM) and 328 g CP/kg (HCM) in isonitrogenous diets (490 g CP/kg). The ingredients and chemical composition of the carbohydrate sources and protein concentrates are presented in Table 1. Using rice bran to mix with the cassava chip increased crude protein content from 24 to 49 g/kg dry matter while fiber content was slightly increased. The two sources of carbohydrate and two levels of cottonseed meal were mixed at four different ratios to be isonitrogenous of 130 g CP/kg (Table 2).
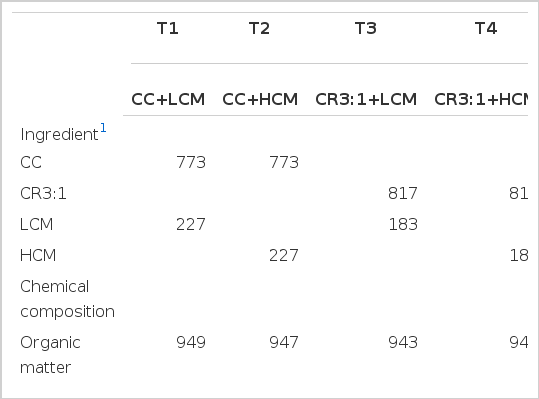
Treatment combinations and chemical composition of concentrate and urea treated rice straw (g/kg DM)
All buffaloes were supplemented with their respective treatment concentrate at 5 g/kg BW divided into two equal feeds (07.00 and 16.00) and urea-treated rice straw was given ad libitum. Mineral blocks and water were available ad libitum for all animals which were housed in individual pens.
Samples and analysis
Measurements of feed intake and collections of feed samples, refusals, feces, rumen fluid, and blood were made during the 21 d of each period. Rumen fluid and blood samples were collected at 0, 2, 4 and 6 h-post feeding while urine and feces were collected via total collection from each individual buffalo during the last 7 d of each period. The samples were stored at −20°C before analysis, while the daily feces collected in each period were bulked, mixed and a 50 g/kg sub-sample taken for later chemical analysis.
Feeds, feed refusals and fecal samples were dried at 60°C and ground (1 mm screen using Cyclotech Mill, Tecator, Sweden) and analyzed using the standard methods of AOAC (1995) for dry matter (DM, ID 967.03), ash (ID 942.05) and acid- detergent fiber (ADF, ID 973.18). Neutral- detergent fiber (NDF) was estimated according to Van Soest et al. (1991) with the addition of α-amylase but without sodium sulphite and the results were calculated with residual ash. Total nitrogen (N) in samples of feeds, feed refusals, and feces were determined according to AOAC (1991) (ID 984.13). Rumen fluid was immediately measured for pH using a portable pH temperature meter (HANNA, instruments HI 8424 microcomputer, Singapore) and NH3-N by Kjeltech Auto 1030 Analyzer (Bremmer and Keeney, 1965). Volatile fatty acids were analyzed using High Pressure Liquid Chromatography (Instruments by controller Water model 600E; Water model 484 UV detector; column Novapak C18; column size 3.9 mm×300 mm; mobile phase 10 mM H2PO4 (pH 2.5)) according to Samuel et al. (1997). Rumen fluid was collected for direct count of protozoa and fungal zoospores using the methods of Galyean (1989) by a haemacytometer (Boeco, Singapore) and bacterial groups (total viable, cellulolytic, proteolytic and amylolytic) were measured using the roll-tube technique of Hungate (1969).
Total urine excretion was collected and acidified using 10 ml of H2SO4 solution (2 M). Urine samples were analyzed for allantoin concentration by high-performance liquid chromatrography, as described by Chen et al. (1993). The supply of microbial N (MN) was estimated by urinary excretion of purine derivatives (PD) according to Chen and Gomes (1995):
Statistical analysis
All data were statistically analyzed according to a 2×2 factorial arrangement in a 4×4 Latin square design using the general linear procedure in GLM procedure of SAS (1996). The statistical model included terms for animal, period, energy source, cottonseed meal level, and interaction between energy source and cottonseed meal level. Unless otherwise stated the significance is reported at p<0.05.
RESULTS
The effects of carbohydrate source and level of cottonseed meal on voluntary feed and nutrient intake are shown in Table 3. It was found that carbohydrate source did not affect total intake, urea-treated rice straw intake or nutrient intakes (p>0.05). However, buffalo fed with HCM had a lower total feed intake (g/kg BW), urea-treated rice straw intake and nutrient intake including organic matter, neutral detergent fiber and acid detergent fiber than buffalo fed with LCM (p<0.05). Moreover, no interaction was found between carbohydrate sources and levels of CM on voluntary feed intake of swamp buffaloes (p>0.05).
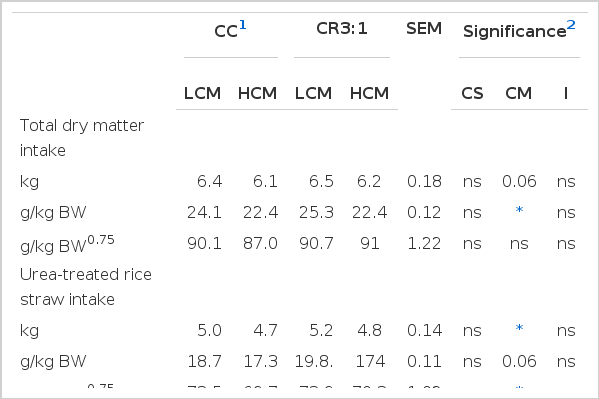
Effect of carbohydrate sources and cottonseed meal levels on total dry matter, urea-treated rice straw and nutrient intakes
Table 4 shows the effect of carbohydrate source and cottonseed meal level on nutrient digestibility and nutrients digested in swamp buffaloes. Carbohydrate source and cottonseed meal level did not affect nutrient digestibility including DM, OM, CP, NDF and ADF of swamp buffalo (p>0.05). Dry matter digestibility was affected by an interaction between carbohydrate source and cottonseed meal level (p<0.05) while digestibility of other nutrients was not significantly different among treatments (p>005). The amount of nutrients digested was not affected by carbohydrate sources (p>0.05), while the amount of DM, OM, NDF and ADF digested were lower in buffalo fed with HCM when compared to buffalo fed with LCM (p<0.05).
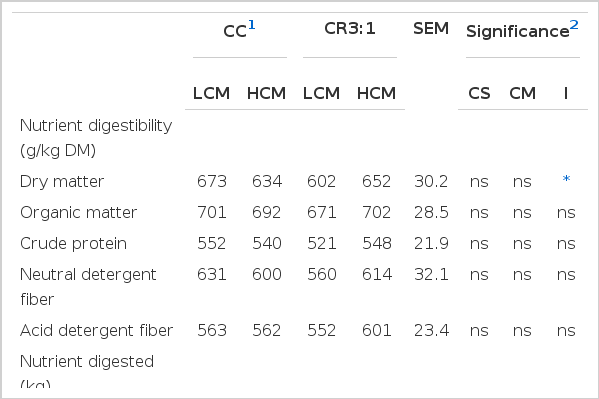
Effect of carbohydrate sources and cottonseed meal levels on nutrient digested and nutrient digestibility
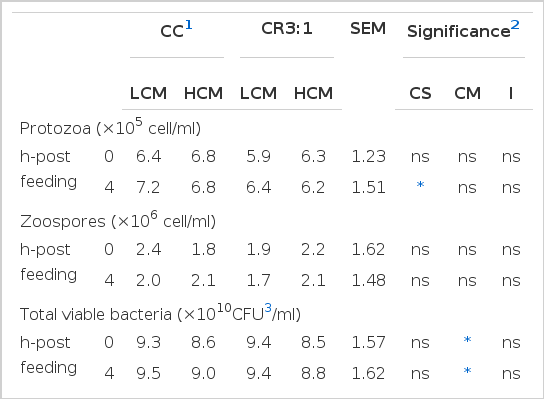
From Table 5, ruminal pH at 0 and 2 h-post feeding in the rumen of swamp buffaloes were similar among treatments (p>0.05). However, buffalo receiving CR3:1 as a carbohydrate source had a higher ruminal pH than the CC fed animals (p<0.05) at 6 h-post feeding while those receiving LCM had a higher ruminal pH than the HCM treatment at 4 h-post feeding (p<0.05). Moreover, ruminal pH at 0 and 2 h-post feeding were affected by carbohydrate source and cottonseed meal level interaction (p<0.05). Carbohydrate sources did not affect ruminal ammonia concentration at any sampling time (p>0.05). Ruminal ammonia nitrogen in the rumen at 4 h-post feeding was lower in buffalo receiving HCM when compared with the LCM group (p<0.05).

Effect of carbohydrate sources and cottonseed meal levels on ruminal pH and ammonia nitrogen concentration
The effect of dietary treatments on the microbial population was that the population of protozoa in the rumen of swamp buffalo was similar among groups before morning feeding (p>0.05). However buffalo which received CR3:1 had lower numbers of protozoa than buffalo fed on the CC treatment (p<0.05). The number of fungal zoospores, measured by the direct count technique, was not affected by either carbohydrate source or cottonseed meal level (p<0.05). Carbohydrate source did not affect viable bacteria numbers as measured by the roll tube technique (Total viable, cellulolytic, proteolytic, amylolytic bacteria; p>0.07), although at 4 h-post feeding the population of amylolytic bacteria tended to be higher in the CC group than in the CR3:1 group (p<0.08). The populations of total viable and cellulolytic bacteria were lower in buffalo fed with HCM (p<0.05) while amylolytic and proteolytic bacteria were not significantly different (p>0.05) to buffalo fed with LCM (Table 6).
Table 7 shows that carbohydrate source did not affect microbial protein synthesis in terms of microbial N supply, microbial protein yield or efficiency of synthesis. Microbial N supply and microbial protein yield were lower in buffalo fed with HCM when compared with LCM (p<0.05). However, CM level did not affect microbial protein synthesis efficiency of swamp buffalo (p>0.05).
DISCUSSION
Carbohydrate source did not affect feed intake or nutrient digestibility. This could imply that both cassava chip and cassava chip combined with rice bran can be used as a carbohydrate (energy) sources in concentrates for swamp buffalo. However, the use of easily rumen degradable carbohydrate feedstuff might be expected to impact on feed intake due to its high rate of volatile fatty acid production and reduction of ruminal pH (Sommart et al., 1997; Richardson et al, 2003; Wang et al., 2009) and the decreased microbial fermentation in the rumen (Firkins, 1996; Russell and Rychlik, 2001; Firkins et al., 2006). The different results from the present study may be due to microbes in the rumen of swamp buffalo were able to adapt themselves to changing substrates (Wanapat et al., 2009). Buffalo fed with HCM had a lower intake of urea-treated rice straw and digested nutrients than those fed with LCM. However, nutrient digestibilities were similar between the two levels of cottonseed meal which could be explained that buffaloes have greater capability to digest mixed substrates, particularly those with low nutritional values (Devendra, 1985; Wanapat and Wachirapakorn, 1990).
Ruminal pH was within the optimum range (6.3 to 6.7) and was found to be similar to other studies (Chanjula et al., 2004; Granum et al., 2007; Wanapat et al., 2009). The differences in ruminal pH between buffalo receiving CC and CR3:1 could be due to the rate of degradation of cassava chip which is higher than rice bran (Chanjula et al., 2003). Digestion of carbohydrate by rumen microbes will produce volatile fatty acids which will influence changes of ruminal pH (Wang et al., 2009). Buffalo which received LCM had higher ruminal pH than the HCM treatment and this could have resulted from the lower ruminal ammonia nitrogen concentration in the HCM than LCM treatments because ammonia nitrogen has property by a base, so this could be one reason why ruminal pH was different among the groups. The concentration of ammonia in the rumen depends on protein degradation which in turn is dependent upon many factors such as the solubility of the protein, the amount of protein in the ration and probably the level of dry matter intake (Church, 1969). In addition, it may be due to differences in ammonia assimilation by bacteria in the rumen (Erfle et al., 1977). Ruminal pH was affected more quickly by carbohydrate source and cottonseed meal levels after morning feeding (0 and 2 h-post feeding). This result indicated that diet feed could improve rumen fermentation by providing suitable pH conditions (6.3 to 7.0) for microorganism activity as reported by Hungate (1966).
Buffalo which received CC had higher numbers of protozoa than buffalo fed on CR3:1. This result is in agreement with Mendoza et al. (1993) and Wanapat et al. (2009) who reported that the number of protozoa decreased when the proportion of starch in the diets decreased as the main source of energy for protozoa particularly Entodiniomoph spp. is starch (Hungate, 1966). However, some reports indicated that grain fed feedlot cattle are virtually free (Eadie et al., 1970; Lyle et al., 1981) or have dramatically reduced populations (Slyter et al., 1970; Vance et al., 1972) of protozoa because of the acidic conditions in the rumen. The population of amylolytic bacteria in the rumen could be increased when ruminants receive a higher proportion of easily rumen degradable carbohydrate (Hungate, 1966; Tajima et al., 2001). However, the population of amylolytic bacteria in the present study only showed a trend towards being higher in the CC than the CR3:1 treatment. This may be due to the capacity of protozoa to store starch granules (Abou Akkada and Howard, 1960), which then reduces the availability of starch for microbial growth (Eadie and Mann, 1970). Fungi may form a significant part of the rumen contents in ruminants fed on fibrous diets and play an important role in fiber digestion (Bauchop, 1979). However, the number of fungal zoospores in this study was not affected by either carbohydrate source or cottonseed meal level. This might be influenced by other factors particularly residence time of feed particles in the rumen (Joblin, 1981), as populations will be rapidly depleted if they are unable to attach to feed particles and passage from the rumen is delayed. Populations of total viable and cellulolytic bacteria were lower in buffalo fed with HCM than with LCM. These results could be a response to roughage intake and fiber intake of buffalo which were higher in the LCM fed animals. This finding is in agreement with Dehority and Tirabasso (1998) who reported that the population of cellulolytic bacteria in the rumen was increased by feeding a diet that contained 550 to 650 g/kg DM purified cellulose although the population of cellulolytic bacteria is not the limiting factor in the digestion of cellulose in the rumen. Proteolytic bacteria numbers were similar between the two levels of cottonseed meal diets while Bach et al. (2005) reported that proteolytic bacteria numbers were reduced when a high proportion of rumen undegradable protein was offered. The different rumen ecology of swamp buffalo from other ruminants could contribute to their different response to different feeds (Wanapat et al., 2003).
Microbial N supply and microbial protein yield were similar between the two carbohydrate sources while they were lower in buffalo fed with HCM than LCM. These results could be due to the feed intake and rumen fermentation responses. Microbial protein leaving the rumen ultimately provides a source of amino acids for the host animal as the protein is digested and absorbed in the small intestine (Church, 1969; Clark et al., 1992). However, there are many factors that affect the amount of microbial protein synthesis such as rate of ruminal degradability of nitrogen and carbohydrate sources, rumen dilution rate, dietary sulfur and frequency of feeding, etc. (Stern and Hoover, 1979). Cottonseed meal level did not affect microbial protein synthesis efficiency of swamp buffalo. As the result shown that proteolytic bacteria and NH3-N concentration not difference by cottonseed meal. This may imply that buffalo which received the LCM diet should obtain more protein from microbial cells than HCM group, but buffalo which received the HCM diet may obtain amino acids from cottonseed meal available in the small intestine directly as this diet contains a high proportion of by-pass protein (Clark et al., 1987). The ability to use cottonseed meal with different sources of energy in a concentrate mixture would be a useful and efficient strategy for farmers who need to find an alternative means to reduce feed costs and a simple way of feeding based on locally available feed resources especially in the developing countries.
Based on the results, it appears swamp buffaloes were able to utilize a higher proportion of rumen fermentable carbohydrate in the diet than young dairy bulls (Wananat et al., 2013) or beef cattle (Wanapat et al., 2012). This could be due to the differences in rumen ecology, especially microbial population, as reported by Wanapat et al. (2003) who found that the ruminal protozoal population was lower, while bacteria and fungal zoospores populations were higher in the rumen of swamp buffalo as compared to cattle. In contrast, use of a high level of rumen by-pass protein dramatically reduced feed intake, nutrient digestibility, bacterial population and microbial protein synthesis of swamp buffalo. It indicated that nitrogen available in the rumen is very important for rumen microbes as Bryant and Robinson (1961) reported that cellulolytic bacteria used NH3-N as a major nitrogen source for their cell protein synthesis. Although by-pass protein is advantageous for the host ruminant to receive good quality protein directly from the diet, nitrogen available for rumen microbes for their growth and cell activities is equally vital and unavoidable (NRC, 2001)
Carbohydrate source did not affect feed intake, nutrient digestibility, rumen fermentation or microbial protein synthesis of swamp buffaloes. Buffalo fed with HCM had a lower roughage intake, population of bacteria and microbial nitrogen synthesis when compared with buffalo fed LCM. Therefore, LCM could be fed with cassava chip as an carbohydrate source in swamp buffaloes.