Effect of Phytogenic Feed Additives in Soybean Meal on In vitro Swine Fermentation for Odor Reduction and Bacterial Community Comparison
Article information
Abstract
The effect of different phytogenic feed additives on reducing odorous compounds in swine was investigated using in vitro fermentation and analyzed their microbial communities. Soybean meal (1%) added with 0.1% different phytogenic feed additives (FA) were in vitro fermented using swine fecal slurries and anaerobically incubated for 12 and 24 h. The phytogenic FAs used were red ginseng barn powder (Panax ginseng C. A. Meyer, FA1), persimmon leaf powder (Diospyros virginiana L., FA2), ginkgo leaf powder (Ginkgo biloba L., FA3), and oregano lippia seed oil extract (Lippia graveolens Kunth, OL, FA4). Total gas production, pH, ammonia-nitrogen (NH3-N), hydrogen sulfide (H2S), nitrite-nitrogen (NO2−-N), nitrate-nitrogen (NO3−-N), sulfate (SO4−−), volatile fatty acids (VFA) and other metabolites concentration were determined. Microbial communities were also analyzed using 16S rRNA DGGE. Results showed that the pH values on all treatments increased as incubation time became longer except for FA4 where it decreased. Moreover, FA4 incubated for 12 and 24 h was not detected in NH3-N and H2S. Addition of FAs decreased (p<0.05) propionate production but increased (p<0.05) the total VFA production. Ten 16S rRNA DGGE bands were identified which ranged from 96 to 100% identity which were mostly isolated from the intestine. Similarity index showed three clearly different clusters: I (FA2 and FA3), II (Con and FA1), and III (FA4). Dominant bands which were identified closest to Eubacterium limosum (ATCC 8486T), Uncultured bacterium clone PF6641 and Streptococcus lutetiensis (CIP 106849T) were present only in the FA4 treatment group and were not found in other groups. FA4 had a different bacterial diversity compared to control and other treatments and thus explains having lowest odorous compounds. Addition of FA4 to an enriched protein feed source for growing swine may effectively reduce odorous compounds which are typically associated with swine production.
INTRODUCTION
Currently, swine production’s great problem is its foul odor. Feces odor is the result of incomplete anaerobic break-down of feed components, particularly peptides in the large intestine (Miner, 1977; Ritter, 1989). The result of this incomplete fermentation is a complex mixture of malodorous substances (Mackie et al., 1998). It is assumed that protein entering the large intestine can be a potential source of odor formation and microorganisms play a central role in the production of these odors (Ritter, 1989). Bacterial fermentation of undigested protein produces toxic substances such as ammonia-nitrogen, amines, VFA, and sulphur compounds (hydrogen sulfide, dimethyl disulfide) (Simpson et al., 1999). The compounds from the protein fermentation products have more unpleasant odor than the carbohydrate (CHO) fermentation products (Claesson et al., 1990). Based upon this information, it is logical to speculate that odors from swine production facilities can be diminished or lessened by reducing odor precursors originating from protein and its metabolites in the digestive tract of animals and in manure stores.
Microbial activities in the large intestine of the animal produce odorous compounds and provide precursors for odor formation in manure. Phytogenic feed additives (FA) are an extremely heterogenous group of feed additives originating from leaves, roots, tubers, or fruits of herbs, spices or other plants which are either available as solid, dried or ground form or as extracts or essential oils (Steiner, 2006). Windisch et al. (2008) concluded that FAs are claimed to exert antioxidative, antimicrobial, and growth-promoting effects in livestock, actions that are partially associated with enhanced feed consumption. Ritter (1989) added that they are one of the biochemical and chemical agents that can reduce odor from animal production facilities. The principle of using FA is to reduce odor formation and emission as well as to alter the microflora in the large intestine of animals and in manure, change the pH into less favorable for odor formation. Thus, altering the microflora and nutrient supply by adding FAs have the potential to change one or more groups of odorous compounds.
There is little information on phytogenic FA using SM as substrate and their effect on the large intestine microbiota. Thus, identifying the suitable FA and nutrient supply which influences beneficial microbial community is essential (Hume et al., 2006; Parker et al., 2007). Denaturing gradient gel electrophoresis-polymerase chain reaction (DGGE-PCR) technique is one of DNA fingerprinting techniques which can be used to describe the microbial diversity of a variety of ecosystems in intestinal microbiota (Muyzer et al., 1993; Felske et al., 1998; Muyzer and Smalla, 1998; Konstantinov et al., 2003; Zhu et al., 2003). Studying the shifts in microbial populations could indicate which microbial species favored the gastrointestinal tract if a particular protein-based ingredient were included in a diet along with particular FA. Using protein as substrate may provide an important influence on the large intestine bacterial community since it is not digested by small intestine enzymes, but reaches the hindgut for fermentation by microorganisms. Therefore, this study evaluated the effect of different FAs using soybean meal as a substrate on reducing odorous compounds in swine by in vitro fermentation and analyzed their microbial communities using DGGE-PCR.
MATERIALS AND METHODS
Experimental design, animals and diets
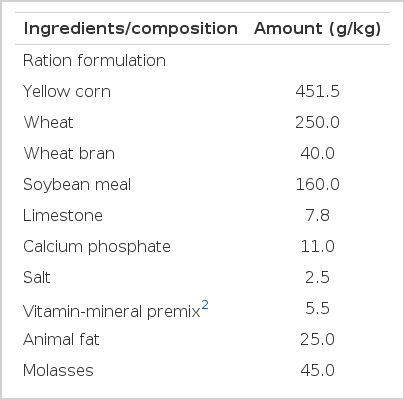
The swine used in this study were selected from a commercial herd, and were the progeny of a Landrace× Yorkshire sow and a Duroc sire. The experimental samples were collected from a total of 9 sixth month old castrated pigs with a live weight of 95±5 kg. The experiment was conducted in the Ruminant Nutrition and Anaerobe Laboratory under the Department of Animal Science and Technology at Sunchon National University (SCNU), Republic of Korea. The experimental proposals and procedures for the care and treatment of the pigs were approved by the Animal Care Committee of SCNU. The animals were housed at the SCNU research farm which was well equipped with water, feed, and disposal systems, and individual pens (12.7 cm×7.6 cm) with fully slatted floors. The diets were fed to the pigs as mash ad libitum in the experimental period. The nutrient composition of the basal diet was in accordance with the suggestions of the nutrient requirements for swine from NRC (1998) are shown in Table 1.
Fresh pig fecal samples were collected directly from the rectum of the swine and transferred to a thermo flask at 39°C under vacuum condition. Collected feces were mixed with 10% (W/V) salt media under a constant flow of CO2 (Jensen et al., 1995; Wang et al., 2004). The homogenate was filtered using sterile cheese cloth and transferred 100 ml of filtrate to sterile serum bottles (120 ml) containing one percent soybean meal (SM) and one tenth percent of phytogenic FA anaerobically O2-free CO2. The bottles were then sealed with sterile butyl rubber stoppers that were kept in place with metal screw caps. The filled serum bottles were subsequently placed in a HB-201SF shaking incubator (HANBAEK Scientific Co., Korea) set at 50 rpm and 37°C in anaerobic incubations for 12 and 24 h.
The phytogenic FAs used were red ginseng barn powder (Panax ginseng C. A. Meyer, FA1), persimmon leaf powder (Diospyros virginiana L., FA2), ginkgo leaf powder (Ginkgo biloba L., FA3), and oregano lippia seed oil extract (Lippia graveolens Kunth, OL, FA4). All treatments and Con were replicated in three. Total gas production, pH, NH3-N, hydrogen sulfide (H2S) and VFA and other metabolites were determined at 12 and 24 h while nitrite (NO2), nitrate (NO3), sulfate (SO4) concentration and PCR-DGGE at 24 h of incubation.
Analysis of odorous compounds
Total gas production (press and sensor machine (Laurel Electronics, Inc., Costa Mesa, CA, USA)), H2S and NH3-N (VRAE Multi-Gas Monitor PGM-7840 (RAE Systems Inc., Sunnyvale, CA, USA)), pH (Pinnacle series M530p meter (Schott Instruments D-55122 Mainz, Germany)), and VFAs and other metabolites (high performance liquid chromatography (HPLC) (Agilent Technologies 1200 series, USA) according to the methods of Tabaru et al. (1988) and Han et al. (2005) were measured from each of the serum bottles at different.
Analysis of indirect compounds
Fermented samples (preserved) were centrifuged at 890×g for 15 min at 4°C and the supernatants were then filtered through 0.2-μm cellulose acetate filters. The filtered supernatants were then used for analysis of nitrite, nitrate and sulfate. The nitrite-N concentration was determined using a Griess reagent kit (G-7921, Molecular Probes (2003)) according to manufacturer’s method. The developed nitrite standard and equation were R2 = 0.9989 and y = 0.1748x+0.0051, respectively. The absorbance of the nitrite was determined using the OD at 548 nm against reference with aforementioned equation. The nitrate concentration was measured based on the OD at 410 nm following the modified method described by (Jenkins and Medsker, 1964). The OD values were calculated from the equation developed from standard solution y = 0.005x–0.0057 (R2 = 0.9973) and values were expressed as mg NO3-N/L. For determination of the sulfate concentration, the modified turbidimetric method conducted according to the method described by (Kolmert et al., 2000) with absorbance at 420 nm. For calculation, the OD values were fitted with an equation, y = 0.0032x+0.0043 and standard, R2 = 0.9911 to obtain an accurate sulfate concentrations.
Microbiological analysis
Fermented samples (preserved) were extracted using Wizard Genomic DNA Purification Kits (Promega, USA). 16S rDNA PCR amplification was performed using 27F and 1492R universal primers. DGGE was performed using a D-Code Universal Mutation Detection System (Bio-Rad, Hercules, CA, USA). To amplify the V3 region of the bacterial 16S rDNA amplicons, 341F-GC and 518R were used (Nübel et al., 1996). Amplicons of the V3 region of 16S rDNA were used for sequence-specific separation by DGGE according to the specifications of Muyzer and Smalla (1998). The DGGE gel was scanned at 400 dpi and similarity indices were calculated for pairs of DGGE profiles. The number of DGGE bands and similarity indices were calculated from the densitometric curves of the scanned DGGE profiles with Molecular Analyst 1.12 software (Bio-Rad) using the Pearson product-moment correlation coefficient (Häne et al., 1993; Simpson et all, 1999) from the Central Microbiology Laboratory of SCNU in Korea.
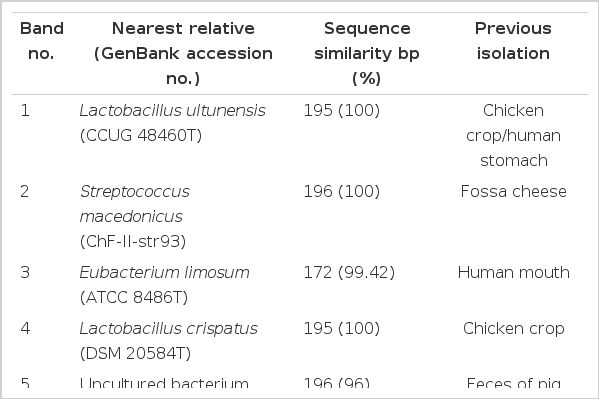
A total of 10 purified dominant bands were sent to Macrogen, Seoul, Korea for sequencing. Results were compared to Gen-Bank database using the BLAST tool of the National Center for Biotechnology Information (NCBI) and EzTaxon.
Statistical analysis
Means of triplicates from treatments and control were analyzed by one-way analysis of variance (ANOVA) using the General Linear Models (GLM) procedures of the Statistical Analysis Systems Institute (SAS, 2002). The effects of the FAs on total gas, pH, NH3-N, H2S, VFA, NO2, concentrations were compared to the controls NO3, and SO4 significant differences between treatment means were examined using Duncan’s multiple comparison tests. Variability in the data was expressed as pooled mean values ±standard error (SE) and significance was declared at p<0.05 for all variables measured.
RESULTS AND DISCUSSION
Effect of FAs on odorous compounds after in vitro fermentation
Predominant formation of malodorous substances occurs in the large intestine due to microbial degradation of several substrates (Jensen and Hansen, 2006). Total gas (TG) production, pH, ammonia-nitrogen (NH3-N), hydrogen sulfide (H2S), nitrite (NO2−), nitrate (NO3−) and sulfate (SO4−−) concentrations of in vitro fermented swine fecal slurry added with soybean meal and different phytogenic feed additives was shown in Table 2. Results revealed that TG production increased as incubation time became longer. FA3 had the highest (p<0.05) total gas production among all treatments and control incubated at 12 and 24 h. This agrees with Robinson et al. (1989) findings wherein TG production is directly proportional to incubation time due to increased fermentation over time. Also, TG was found higher compared to our previous results obtained wherein the FAs added but starch was used as substrate (Alam et al., 2012). Moreover, result was also partially similar to Patra et al. (2006) results in which total gas production as well as odorous compounds increased within the duration of incubation period. However, increase in gas production is not an indicator of odor intensity because it depends on what types of odorous compounds are produced.
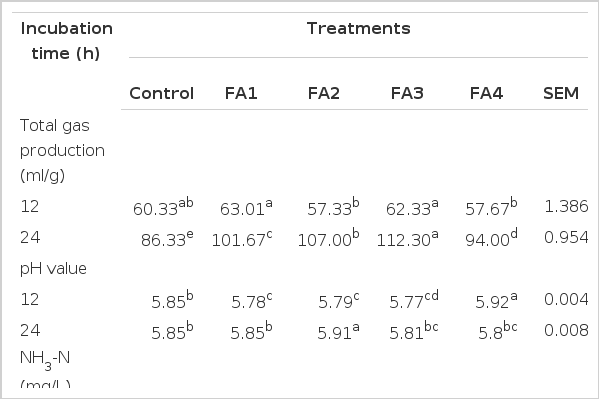
Total gas production, pH, ammonia-nitrogen (NH3-N), hydrogen sulfide (H2S), nitrite-nitrogen (NO2−-N), nitrate-nitrogen (NO3−-N) and sulfate (SO4−−) concentrations of in vitro fermented swine fecal slurry added with soybean meal and different phytogenic feed additives
The pH values on all treatments increased as incubation time became longer while FA4 decreased. The current result obtained on pH value was opposite of our previous research obtained in Alam et al. (2012). This is due to different substrate used during in vitro fermentation. Reduced pH of FA4 from 12 to 24 h incubation was supported by Risley et al. (1992) and Kempen (2001), stating that lowering the pH of urine and subsequent slurry can be beneficial for reducing odor and NH3-N emissions. Maintaining the proper acid-base balance and buffering capacity of the diet and the intestinal contents may influence the final pH. In addition, Bailey et al. (2002) stated that anaerobic incubation with additive added fermenta caused significant time-dependent decreases in pH from 0 to 24 h. This trend of lowering pH is probably beneficial when it comes to reducing odorous compounds.
NH3-N concentration of FA2 was lowest (p<0.05) after 12h of incubation followed by FA3, FA1 and control, respectively while FA4 was not detected. Moreover, only control was detected with NH3-N concentration after 24 h of incubation. Naidu et al. (2002) stated that a novel probiotic effectively reduced sulfur and NH3-N compounds in vitro. NH3-N gas concentration reduction might be due to the antimicrobial effect of the FA. Macfarlane and Macfarlane (2003) showed that NH3-N and odorous branched chain fatty acids are produced from the fermentation of amino acids. Killeen et al. (1998) reported that FAs contain subfractions which have partially antagonistic properties on intestinal urease activity and NH3-N formation under in vitro conditions. In addition, Duffy et al. (2001) confirmed the existence of active components in extracts which lowered or reduced intestinal urease activity and enzymes involved in the metabolic urea cycle.
H2S concentration was not detected in FA2, FA3 and FA4 at 12 and 24 h of incubation. Meanwhile, FA1 H2S concentration was only detected at 12 h but it was detected at 12 and 24 h incubation in control. FA2 to FA2S. Fakhoury et al. (2000) stated that H2S had the highest correlation of malodorous sulphur compounds which may be reduced by adding selective FAs to the diet. Moreover, Le et al. (2005) reported that a change in pH may also change the release of other odorous compounds such as hydrogen sulfide. The decreased in pH of FA4 explains the reduced to undetectable amounts of H2S.
NO2-N concentration was better in FA supplemented groups than control while NO3-N concentration was better in FA4. After 24 h of incubation, NO2-N and NO3-N concentration was highest (p<0.05) FA4. NH3-N is nitrified by bacterial enzymes and converted to NO2 and NO3. Ward (1996) explained that oxidation of NH3-N to NO3 is called nitrification which is the result of actions of the nitrosifyers (NH3-oxidizing, NO2 producing bacteria) and nitrifying bacteria (NO2-oxidizing, NO3 producing bacteria). Bengtson (2010) also showed the NH3-N, NO2, and NO3 correlation and their conversion through N-cycle. This explains that the conversion of NO2 or NO3 from NH3-N is beneficial for odor reduction during incubation period. FA4 might be a good FA for reducing nitrogen compounds through conversion to NO2 and NO3.
Evaluation of SO4 production is an indirect method used to evaluate H2S. In the metabolic pathway, the conversion of H2S to SO4 is referred to as chemolithotrophic oxidation of sulfur (Kelly, 1982) is favorable to odor reduction; therefore, higher SO4 production is better. SO4 concentration was highest (p<0.05) in FA4, FA1 and FA3 followed by FA2 and control, respectively. Hao et al. (1996) reported that sulfur-containing compounds are produced by bacteria through two processes: reduction of SO4 and metabolism of sulfur-containing amino acids. SO4 reduction proceeds via either assimilatory or dissimilatory pathways. In the first process, bacteria produce enough reduced sulfur for cell biosynthesis, while in the second process SO4 is utilized as a terminal electron acceptor and large quantities of sulfide are produced. It was observed in the present experiment that FA treatments were more effective in reducing H2S to SO4 than control at 24 h.
Gibson et al. (1988) stated that SO4-reducing bacteria (SRB) may play an important role in the terminal stages of fermentation in the colon and in particular, their ability to utilize hydrogen can prevent excessive amounts of this gas from accumulating in the colonic lumen. FA added in this experiment might help in the stimulation of those beneficial bacteria, such as sulfate-producing bacteria which reduces the sulfide. This effect might be due to FA’s enhancement of beneficial microbes and anti-microbial action against undesirable microbes (Spoelstra, 1980). Based upon our findings, we confirmed that selected FA groups produced more SO4 than control. So, FA4 as well as the other FAs are suitable to reduce H2S by converting it to SO4.
Jensen and Hansen (2006) and Le et al. (2005) explained that the predominant formation of malodorous substances occurs in the large intestine due to microbial degradation of several substrates or residual feed components. Substrate fermentation depends on microbial activity which may be modified by antimicrobial FAs. VFA and other metabolites production of in vitro fermented swine fecal slurry added with soybean meal and different phytogenic feed additives were shown on Table 3. Formate was only detected in 12 h FA4 in vitro fermenta. Lactate production was highest (p<0.05) in FA3 but propionate and butyrate were found highest (p<0.05) in control and FA2 after 12 h of incubation, respectively. After 24 h of incubation, control has the highest (p<0.05) in lactate and propionate production while FA3 has the highest (p<0.05) butyrate production. Total VFA production was found highest (p<0.05) in FA2 followed by FA3, FA4, FA1 and Con, respectively after 12 h incubation while after 24 h incubation, FA3 was found highest (p<0.05) followed by FA2, FA4, FA1 and Con, respectively. van Beers-Schreurs et al. (1998) explained that the quantity of VFA depends on the amount and composition of the substrate and on the type of microbes present in the cecum. Low VFA and other metabolites concentration indicates a low amount of substrate fermented and microbial activity in the cecum of pigs.
Bacterial communities of in vitro fermenta added with different FAs
Negative image and similarity index of 16S rRNA DGGE amplified using total genomic DNA extracted from in vitro fermenta of swine fecal slurry added with different phytogenic feed additives was shown in Figure 1. These bands represented dominant species in the community that were enriched by SM and respective FAs. There were three clearly different clusters: I (FA2 and FA3), II (Con and FA1), and III (FA4). The similarity indices in profiles of clusters I, II, and III, were 88%, 100%, and 36%, respectively. FA4 had the lowest similarity among communities. In the previous research of Alam et al. (2012), the same FAs were added using starch as substrate but the bacterial community and similarity index were different from this research. This means that the substrate affects the bacterial community, similarity indices as well as identified dominant bands. FAs provide a subfraction for the bacteria that can utilize SM and affects their colonization of the large intestine as in vitro. The similarity index covered the range of different percentages for the clusters. The different ranges of the three clusters indicate that different FAs had an effect on the microbial communities during in vitro fermentation after 24 h of incubation. This supports the idea that different FAs create a strong selection pressure on the microbial community in vitro. Among the three clusters, cluster III (FA4) had the lowest similarity (36%) which was evidence of a different microbial community. It has been shown that some FAs can beneficially affect the host by selectively stimulating the growth and or activity of one or a limited number of bacteria in the intestine or gut (Tannock, 1999). Overall, different clusters indicate that the bacterial communities were changed with the addition of FAs which might influence the intestinal bacterial communities involved in fermentation. Our results are consistent with the in vitro fermentation parameters and the concepts of Mao et al. (2008).
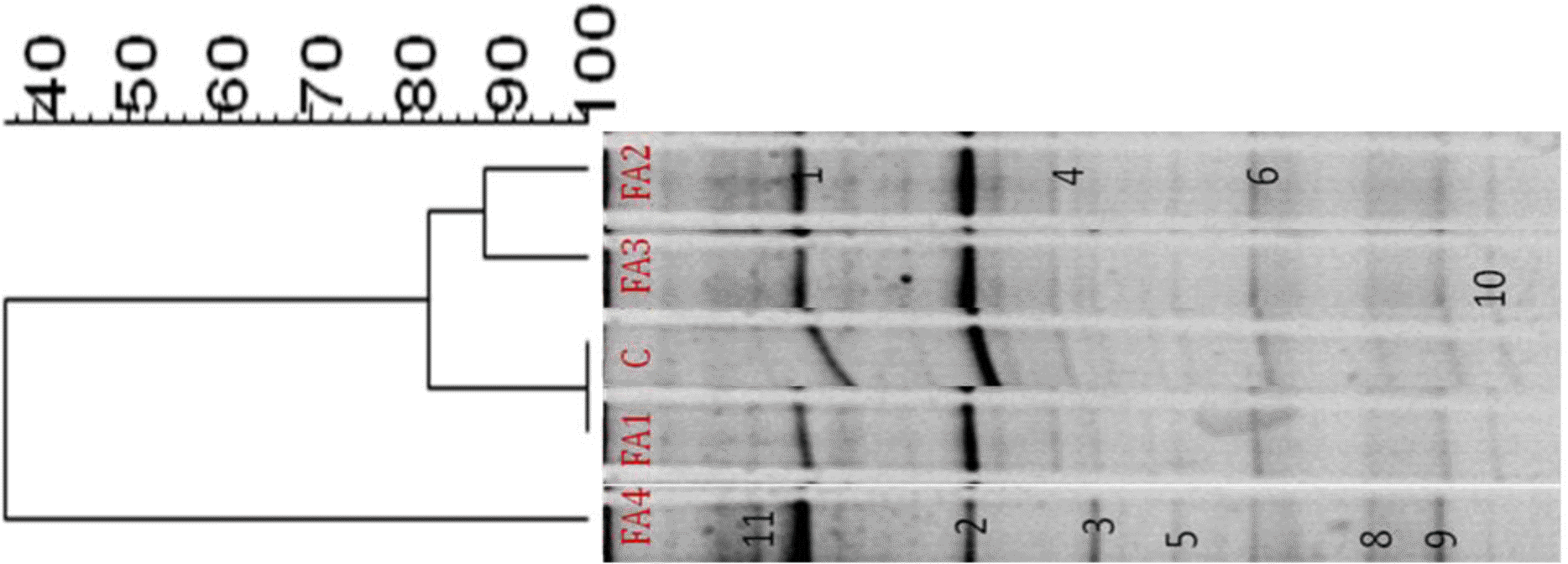
Negative image and similarity index of 16S rRNA DGGE amplified using total genomic DNA extracted from in vitro fermenta of swine fecal slurry added with different phytogenic feed additives. C: control; FA1, red ginseng barn powder; FA2, persimmon leaf powder; FA3, ginkgo leaf powder; FA4, oregano lippia seed oil extract (OL).
Identified dominant bands from 16S rRNA DGGE amplified using total genomic DNA extracted from in vitro fermenta of swine fecal slurry added with different feed additives was shown in Table 4. Identity ranges from 96 to 100% sequence similarity with 170 to 196 bp. Most of the identified bands were previously isolated from different digestive parts of different animals. Identified dominant bands were almost similar our previous result obtained (Alam et al., 2012). This is due to the same FAs added which obviously enhanced some bacterial species during fermentation process. There were three dominant bands which were present only in the FA4 treatment group and were not found in other groups. They were identified closest to Eubacterium limosum (ATCC 8486T), Uncultured bacterium clone PF6641 and Streptococcus lutetiensis (CIP 106849T). The three distinct strains present in the FA4 fermented group probably modified the fermenta environment in some way, either utilizing or enhancing the odor reducing bacteria. FA4 had different bacterial diversity compared to control and other treatments and thus explains having lowest odorous compounds. However, the threshold for similarity to be considered as the same species is 97%, according to Stackebrandt and Goebel (1994); therefore, it appears that the sequence of band no. 5 with similarity of <97%, likely represents some different, not yet cultured pig intestinal or fecal bacteria, that has not previously been identified. This is similar to the situation in the human intestine, where most species analyzed by DGGE and sequencing have not been previously identified (Zoetendal et al., 1998). FAs may provide a subfraction for bacteria that can utilize odor compounds, and these odor compound-utilizing bacteria may then accelerate the metabolism of the other intermediate products such as NO2, NO3 and SO4. The reported substantial reduction effect of certain FAs compared to others can be explained by the fact that it elicits detrimental effects on the microbial activities which release malodor from swine manure as well as function as masking agents (Varel and Miller, 2001). Currently, the precise mechanism of how the above additives reduce the odorous compounds is not well documented. A suggested explanation reported by Kim et al. (2008) is a transformation of the microbial species related to odor generation in the slurry. It may be due to changes in pH, characteristics of the substrates, and microbial activities. FA4 of present experiment seems to function similarly. Moreover, herbs and spices are known to exert antimicrobial actions in vitro against important pathogens, including fungi (Özer et al., 2007). The antimicrobial mode of action is considered to arise mainly from the potential of the hydrophobic essential oils to intrude into the bacterial cell membrane, disintegrating membrane structures, and causing ion leakage. As such, FAs may also be beneficial to swine by reducing pathogens along with odor reduction.
CONCLUSIONS
OL generated a minimum of odorous compounds during in vitro fermentation. OL may be effectively used as a FA with protein-rich conditions in the large intestine to reduce the odor in growing swine. Moreover, selection of intestinal microbial species by a suitable FA in the in vitro conditions could provide a means to select or detect one or more bacterial species that could be appropriate for use as probiotics, either alone or in combination with animal diets. However, further research is needed using recommended FAs to determine whether these effects can also be detected in vivo at different ages factors along with CHO enrichment. Alternatively, real-time PCR using genus or strain-specific primers could be used to quantify the number of identified bacteria.
Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ0074512012)” Rural Development Administration, Republic of Korea.