The Effects of Pre-slaughter Stress and Season on the Activity of Plasma Creatine Kinase and Mutton Quality from Different Sheep Breeds Slaughtered at a Smallholder Abattoir
Article information
Abstract
The objective of the current study was to determine the effect of pre-slaughter stress, season and breed on the activity of plasma creatine kinase (CK) and the quality of mutton. One hundred and seventy-three (173) castrated sheep from Dormer (DM), South African Mutton Merino (SAMM), Dorper (DP) and Blackhead Persian (BP) sheep breeds were used in the study. The animals were grouped according to age-groups as follows: Group 1 (6 to 8 months), Group 2 (9 to 12 months) and Group 3 (13 to 16 months). Blood samples were collected during exsanguinations using disposable vacutainer tubes for CK analysis. Representative samples of the Muscularis longissimuss thoracis et. lumborum (LTL) were collected from 84 castrated sheep, of different breeds (28 per breed) 24 h after slaughter. The following physico-chemical characteristics of mutton were determined; meat pH (pH24), color (L*, a* and b*), thawing and cooking losses and Warner Braztler Shear Force (WBSF). The activity of plasma CK was significantly higher (p<0.001) in summer (1,026.3±105.06) and lower in winter (723.3±77.75). There were higher values for L* (33.7±0.94), b* (11.5±0.48) and WBSF (29.5±1.46) in summer season than in winter season; L* (29.4±0.64), b* (10.2±0.33) and WBSF (21.2±0.99). The activity of plasma CK was influenced by the type of breed with Dormer having the highest (p>0.001) levels (1,358.6±191.08) of CK. South African Mutton Merino had higher values for pH24 (5.9±0.06), L* (34.2±0.97), b* (12.2±0.50) and WBSF (26.8±1.51) and Blackhead Persian had higher values (35.5±2.17) for cooking loss (CL%) than the other breeds. Computed Principal Component Analyses (PCA) on the activity of plasma CK and physico-chemical characteristics of mutton revealed no correlations between these variables. However, positive correlations were observed between pH24, L*, a*, b*, CL% and WBSF. Relationships between pre-slaughter stress, CK activity and physico-chemical characteristics of mutton were also observed. It was therefore concluded that although mutton quality and creatine kinase were not related, pre-slaughter stress, season and breed affected the activity of creatine kinase and mutton quality.
INTRODUCTION
Sheep are important in the communal areas of Eastern Cape, South Africa as they are a source of cash, milk, wool and meat (Mapiliyao et al., 2012). The province has the highest (30%) meat consumption percentage of meat that is consumed locally (DAFF, 2010). Of the 95 abattoirs found in Eastern Cape Province of South Africa, more than 50% of them are classified as smallholder (low throughputs) abattoirs (ECRMA, 2012). Thirty percent of the meat that is consumed locally comes from the smallholder abattoirs. However, not much work has been documented about the quality of mutton produced under practical conditions with reference to pre-slaughter stress. Information that relates to the effect of pre-slaughter stress on mutton quality has been conducted under experimental designs (Lowe et al., 2001; Miranda-de la Lama et al., 2010a). Pre-slaughter stress is caused by the activities such as the period of collecting the animals in preparation for loading at the farm, loading and off-loading, transportation of animals to the abattoir and lairage period at the abattoir (Adzitey, 2011). It is influenced by the animal’s previous experiences and specific features observed at the farm and at the lairages (Muchenje et al., 2009; Dodzi and Muchenje, 2011; Ndou et al., 2011).
Stress reactions are adaptive; they help the animal to cope with the novelty of the environment by inducing physiological and behavioral changes (lactate, glucose, plasma creatine kinase “CK” and cortisol) in the animal (Terlouw, 2005; Gregory, 2010). Some of the pre-slaughter stress factors like ambient temperature, transportation, distance and lairage duration (Adzitey, 2011) leads to fatigue (Warriss et al., 1998), cause animals to release enzymes and hormones (creatine kinase, cortisol and catecholamines (De Haan et al., 1995; Terlouw, 2005; Muchenje et al., 2009) into the blood stream leading to a series of secondary processes that involve energy metabolism, respiratory function, immune function and osmotic regulation (Hoffman et al., 1998; Ali et al., 2006). Fatigue can be observed in sporting activities, but in animals it can be induced by repeated short contractions of the muscle which causes internal muscle damage. These internal muscle damages are difficult to identify in sheep due to the long and soft wool they possess (Kannan et al., 2000; Ali et al., 2006). Furthermore, fatigue results in the depletion of glycogen reserves in the muscles causing an increase in lactic acid production post mortem, which subsequently increases meat pH (pH24) (Terlouw and Rybarczyk, 2008; Veiseth-Kent et al., 2010).
Increased use of ATP give rise to the activity of the enzyme called creatine kinease; CK (Allen et al., 1995). Creatine Kinase is found in the skeletal muscles of animals, responsible for maintaining energy homeostasis at the sites of high Adenosine TriPhosphate (ATP) (Dieni and Storey, 2009). The activity of CK found in plasma or serum is used to diagnose muscular damage in many species (Hamburg et al., 1991; Hornikova et al., 2009). When CK is found in the blood plasma, it indicates muscle damage (Sáncheza et al., 1999). Rupture of muscles causes the release of CK which is deposited into the blood (Vojtic, 2000). Total activity of CK can also be used to measure the sum of enzyme activities from different organs of the animal. It was noted that CK can either be an M or a B monomer subunit. Muscle creatine kinase (M-CK) which can also be denoted by S-CK is found in the M-line of the H-zone of the sarcomere length (Brancaccio et al., 2007; Hornikova et al., 2009). Therefore, the presence of CK in the blood indicates muscle exertion by adverse conditions and poor sheep welfare during the pre-slaughter period (Tadicha et al., 2005).
Pre-slaughter stress such as loading and bullying of animal by others (animal characteristics) has a negative impact on the eating quality of meat (Vimiso and Muchenje, 2013) such as pH, color, thawing loss, cooking loss and WBSF values (toughness of meat). Animal-related characteristics include breed, age, gender and health status of the animal (van Heerden et al., 2007; Sainsbury et al., 2011). Hopkins and Fogarty (1998), Toohey and Hopkins (2006) and Rodríguez et al. (2011) reported that diverse lamb genotype, fattening of suckling lamb under commercial and controlled conditions have a negative effect on the carcass quality and eating quality of mutton. However, there is limited information regarding the activity of CK and physico-chemical characteristics of mutton as affected by pre-slaughter stress, season and type of breed under practical conditions. Therefore, the objective of the current study was to determine the effect of pre-slaughter stress, season and type of breed on the activity of CK and the quality of physico-chemical characteristics of mutton from different sheep breeds slaughtered at a smaller holder abattoir.
MATERIALS AND METHODS
Description of the study
The study was carried out at Adelaide Nxuba local municipal abattoir located 32°8′ S and 26°9′ E in the Amathole District of the Eastern Cape Province, South Africa. Adelaide is located at 586 m above sea level. It is also situated in the semi-arid False Thornveld of the Eastern Cape. The temperatures in Adelaide during the period of study were ranging from 15°C to 36°C with mean temperatures of 21.5°C.
Study animals
The study was conducted under practical conditions at a small-holder municipal abattoir. Data were collected as animals were brought in for slaughter. No attempt was made to control the activities happening during process; this includes both pre-slaughter stress and animal-related characteristics (breed, age and sex). One hundred and seventy-three (173) sheep that were presented for slaughter at the abattoir between winter (March – August 2010) and summer (September – March 2011) seasons were used. Among those slaughtered, 4 different breeds (Dormer, South African Mutton Merino, Dorper and Blackhead Persian) of sheep were identified. The number of animals per breed was as follows; 15 Dormer, 46 South African Mutton Merino, 77 Dorper and 35 Blackhead Persian. The animals used were castrates with overall slaughter weight of approximately 39.5 to 40 kg. The animals were brought to the abattoir for slaughter once they attained weights varying between 39.5 or 40 kg.
Transportation
The animals were transported by light duty vehicles from different farms to the abattoir. The average size of each vehicle was 3.6 m×4.4 m with the stocking density of 0.75 m2, approximately 3 units of sheep (during the summer season) to 4 units of sheep (during the winter season). One unit of sheep is equivalent to 5 sheep, therefore in summer 15 animals were transported per load and in winter 20 animals were transported per load. The stocking density was calculated by dividing the floor area by the number of animals in the vehicle and the density was expressed as animals/m2. Departure time from the farm, distance travelled and lairage duration were recorded. On arrival at the abattoir, sheep were given water ad-libitum prior to slaughter. The average daily temperature after transportation was obtained from Adelaide town weather records. A record sheet was used to capture all the records.
Biochemical/physiological determination of blood
Blood collection and plasma separation:
An electric stunner of 650 Volts was used for five seconds to make the sheep are unconscious. The sheep were suspended by their hind leg and a sharp knife was used to cut the throat to allow bleeding. They were allowed to bleed for 6 min. During exsanguination blood was collected from each sheep while they were still hanging, after cutting of the throat. Disposable vacutainer tubes containing anticoagulant Ethylenediaminetetraacetic acid (EDTA) were used to collect blood samples. Blood sample from each animal was labeled accordingly and kept in ice until plasma was separated within 2 h after collection. Blood samples were centrifuged at 21°C for 10 min at 3,550 rpm placed in 1.5 mL Eppendorf tubes and stored at −20°C (Model 5403 Centrifuge, Gatenbay Eppendorf GmbH, Engelsdorp, Germany) to separate plasma from the blood. The samples were then arranged in a rack and marked to correspond to the type of breed it was collected from.
Creatine Kinase (CK) activity determination:
The activity of plasma CK was analyzed at National Health Laboratory Services, Port Elizabeth (South Africa) using Model DXC 600 machine (Beckman Coulter, Ireland) with reactive ingredients for SYCHRON Systems (CK 2* 200). The ingredients were added for quantitative determination of CK activity of units per liter (U/L) in plasma. The contents included 2* 61ML CK Reagent, 1 Preparation insert and the reactive ingredients included Creatine Phosphate, Disodium Salt 461 mmol/L, Nadide 30:0 mmol/L, Adenosine-5-Diphosphate, Monopotassium Salt, Dihydrate 36.0 mmol/L, Glucose 24.0 mmol/L, Glucose-6-Phosphate Dehydrogenase 46.1 kU/L and Hexokinase 136 kU/L.
Meat quality measurements
Representative samples of the Muscularis longissimuss thoracis et. lumborum (LTL) were collected from 84 castrated sheep of different breeds (28 South African Mutton Merino, 28 Dorper and 28 Blackhead Persian). No meat sample was collected for Dormer since the owner of the animals wanted the whole carcass without any cuts from the animals where meat samples were collected. The muscle was removed by cutting a sample between the 4th and 6th ribs (14×9×3 cm) of the loin region while the carcasses were still hanging. The collected samples were used for the measurements of pH24, color (L*, a*, and b*), thawing and cooking losses and Warner Braztler Shear Force (WBSF) of meat. The weight of the meat samples ranged from 191 to 210 g. Each sample was vacuum-packed, kept in a cooler box an hour after collection and further stored in the refrigerator at −4°C.
pH measurements:
A portable pH meter, with a fibre-optic probe (CRISON pH 25 Instruments SA, Alella, Spain) was used to measure pH of the meat 24 h post mortem. It was measured from the Muscularis longissimuss thoracis et. lumborum muscle removed from the hanging carcasses. The pHu meter was first calibrated using pH 4, pH 7 and pH 9 standard solutions (CRISON Instruments, SA, Spain). The measurements were then performed with a sharpened metal sheath to prevent probe breakage. The values of pH24 were recorded accordingly.
Determination of meat color:
The color of meat (L* = lightness, a* = redness and b* = yellowness) (Commission International De I’ Eclairage, 1976) was determined at the butchery 24 h after slaughter from the muscle samples removed on the hanging carcasses and transported from the abattoir. A portable refrigerator vehicle was used to move carcasses from the abattoir to the butchery an hour after slaughter. After cutting the sample from the carcass, a Minolta color-guide 45/0 BYK-Gardener GmbH machine with a 20 mm diameter measurement and illuminant D65-day light, 10° standard observer was used for color measurement. The machine was calibrated each day before taking measurements using the green, black and white standard color samples provided for this purpose. The readings were taken by rotating the Color Guide 90° between measurements so as to obtain the average value for the color.
Determination of WBSF, thawing and cooking losses of mutton:
On average, after 21 d, the Muscularis longissimuss thoracis et. lumborum muscle samples were removed from refrigeration, weighed, then thawed and weighed again. The recorded weight differences were expressed as the thawing loss using the following formulae:
The meat samples were placed in a plastic bag and cooked using water bath at 85°C for 45 min (Ding et al., 2010). Cooking loss was then calculated using the following formulae:
Tenderness of mutton was determined using the Instron-Warner-Bratzler Shear Force (WBSF) machine. After cooking, 3 sub samples of specified 10 mm core diameter were cored parallel to the grain of the meat. The samples were sheared perpendicular to the fiber direction using a Warner Bratzler (WB) shear device mounted on an Instron (Model 3344) Universal Testing apparatus (cross head speed at 400 mm/min, one shear in the centre of each core). The mean of maximum load (N) was recorded for each batch.
Statistical analysis
Generalized Linear Models procedure of SAS (2003) was used to determine the effect of breed, age and season on CK activity. The following model was used:
Where,
yijk
Response variable (CK activity and mutton quality characteristics)
μ
Overall mean,
αi
Breed effect (Blackhead Persian, Dorper, Dormer and SA Mutton Merino breeds),
βj
Seasonal effects (summer and winter season),
ɛijk
Random error term.
Pair-wise comparison of means was done using the LSD method. The relationship between continuous variables, CK and physico-chemical characteristics of mutton were analyzed using PROC Regression model of SAS (2003) to determine the CK and physico-chemical characteristics of mutton. The following model was used:
Where,
y
Response variable (CK, pH24, L, a*, b*, Thaw %, CL% and WBSF),
a
Intercept,
b
Co-efficient variable,
X
Continuous variables (Temperature, Transport time, Lairage duration and Stocking density. The Principal Component Analysis (PCA) was also used to determine the relationship between CK and physico-chemical characteristics of mutton.
RESULTS AND DISCUSSION
Effect of season on the activity of CK and physico-chemical characteristics of mutton
Table 1 shows the effects of season on the activity of plasma CK and physico-chemical characteristics of mutton. An increased (p<0.001) CK activity (1,026.3±105.06) was observed in summer than in winter season. During the summer season, CK production increased as a result of the rapid respiration rate of the animal. In addition, increased levels of CK could be a result of physical activity at high temperatures that leads to muscular damage, including fatigue due to prolonged and intense muscle activity. Similar results were found by Kannan et al. (2000); Miranda-de la Lama et al. (2010a) and Miranda-de la Lama et al. (2012) where higher CK activity was as a result of stress associated with transportation during summer season in goats and lambs. The increase in CK could be attributed to an increase in ATP demand that is used to speed up respiration and metabolism during pre-slaughter stress (Chulayo and Muchenje, 2013). Pollarda et al. (2002) reported that, elevated activity of CK in red deer was due to transportation. However, lower CK activity in goats was reported by Zimerman et al. (2011) due to the summer season and adverse pre-slaughter conditions. During transportation, muscle enzyme activity increases as a result of increased muscle cell permeability or muscle cell damage. This makes CK a sensitive indicator of muscular damage and fatigue (Miranda-de la Lama et al., 2010b).
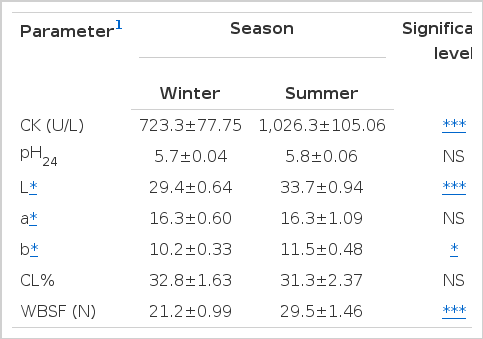
Least square mean values (±SE) for creatine kinase, ultimate pH, color and cooking loss % and WBSF of mutton as affected by season for all breeds
In Table 1, L* (p<0.001), Warner Bratzler Shear Force (WBSF) (p<0.001) and b* (p<0.05) were affected by season because of the abrupt change in environmental temperatures in winter that lead to dark cutters. Seasonal changes have been reported to have a negative effect on the quality of pork (Rodríguez-Sánchez et al., 2009). It was also reported that season has an effect on mutton quality (María et al., 2006). Adverse pre-slaughter stressors are also known to negatively affect meat quality attributes such as color, tenderness and pH24 (Mazzone et al., 2010; Miranda-de la Lama et al., 2012). However, there were no significant (p>0.05) effects of season (Table 1) on pH24, redness (a*) and cooking loss % (CL%) in the current study. Dalla Costa et al. (2007) reported negative impact of the winter season on the quality of pig meat.
Pre-slaughter stress on the activity of CK and physico-chemical characteristics of mutton
Table 2 shows a relationship between pre-slaughter stress and the activity of CK and physico-chemical characteristics of mutton. A positive linear relationship was observed between temperature and CK activity. The study also concurs with the findings of Melesse et al. (2011) who reported increased CK activity due to high temperatures. Partida et al. (2007) also reported that increasing environmental temperatures and pre-slaughter stress caused by loading, transportation and lairage duration also increased CK activity. According to Earley et al. (2012), longer hours of transportation are also a source of stress and it increases CK activity in the blood. During transportation, the truck stocking density did not have an effect on the animal since there was no muscle injury associated with stocking density. Miranda-de la Lama et al. (2012) reported fewer/no injuries associated with stocking density. However, in a moving truck and low stocking density, animals require more energy in order to keep themselves balanced. There was no relationship observed between pre-slaughter stressors such as distance, lairage duration, stocking density and enzyme CK activity.

Pre-slaughter stress (temperature, stocking density, lairage and distance duration) and their relationship with creatine kinase activity and physico-chemical characteristic of mutton
A positive and negative linear relationship between temperature, distance and lairage duration on pH24 and L*, stocking density, lairage duration and temperature on pH24 and b* and between distance duration and a* were observed. Pre-slaughter stress may result in lower glycogen levels which may lead to increased pH values from meat causing darker meat (Muchenje et al., 2008; Yu et al., 2009). The color of mutton is as a result extrinsic factors such as pre-slaughter conditions, slaughtering itself and oxygenation and oxidation processes during ageing which later affect color evolution (Jose et al., 2008). Increase in transportation time resulted in a decrease in lightness and yellowness of meat while redness and pH24 increases. Animals endure pain during long journeys because of fasting and exhaustion towards the end of the journey (Abril et al., 2001; Van de Water et al., 2003). Similarly, Lambertini et al. (2006) reported that the meat from rabbits was affected by pre-slaughter lairage duration and transportation. In addition, L* and b* are reduced while a* values increases.
The effect of breed on the activity of CK
Table 3 shows significant breed effect on the activity of CK. Among the sheep breeds, Dormer had the highest activity of CK (1,358.6±191.08) than Blackhead Persian (726.2±117.08), SA Mutton Merino (758.0±107.47) and Dorper (656.3±81.79). Dormer is a breed from Dorset Horn and Germany Merino bred to adapt to winter rainfall conditions of South Africa. Nevertheless, these breeds are indigenous breeds that can be raised under harsh conditions except for Dormer which is sensitive to rough handling. The hardiness of Dorper could also be indicated by the reduced CK activity that made it to withstand poor pre-slaughter conditions (Fourie et al., 2002). Even though these sheep are indigenous, BH is a fat-tailed breed whereas SAMM produces wool and meat (Almeida, 2011). Therefore, Dorper can be slaughtered during the summer months as it survives even under harsh conditions.
The effect of breed on physico-chemical characteristics of mutton
Table 4 shows the physico-chemical characteristics of mutton from the three breeds. Mutton from South African Mutton Merino had higher (p<0.001) values for pH24, L* and b* than Dorper and Blackhead Persian sheep The differences observed between BP, DP and SAMM breeds for pH24 were anticipated because they are meat producing breeds even though they have other traits of economic importance such as for wool (SAMM) and fat (BP) production (Schoeman, 2000). In addition, the results obtained in the current study could be that SAMM is a late maturing breed as compared to DP (Webb and O’Neill, 2008). This is also due to the fact that type of breed is one of the factors that cause variation in meat quality (Sanudo et al., 1996). The results were not comparable to those of Teixeira et al. (2005) who found no differences on meat at 24 h after slaughter. However, Cloete et al. (2008) found difference in pH24 when comparing Merino breed with other breeds of sheep. The pH24 from DP and SAMM were above the normal range of pH 5.4 and 5.7 (Hoffman et al., 2003) making the meat undesirable.
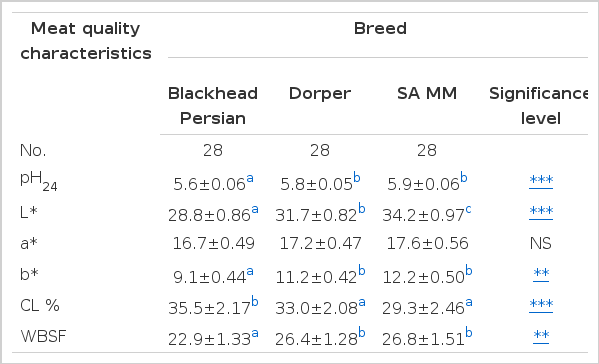
Mean (±SE) for pH24, L*, a*, b*, CL% and WBSF of mutton as affected by Blackhead Persian, Dorper and SA Mutton Merino
Higher levels of pH24 affect the conversion of muscles to meat because of reduced glycogen reserves and low lactic acid in the muscles after slaughter (Ferguson and Warner, 2008; Rodríguez et al., 2011). The pH24 of muscles is one of the contributing factors to the quality of meat because increased ultimate pH (pHu) results to darker meat. The color of meat is what consumer use to charge the quality of meat and make purchasing decisions (Rani et al., 2013) extremely important indicator of red meat quality, smell and texture were regarded as more important quality indicators (Radder and Le Roux, 2005). It was also reported by Mach et al. (2008) that beef with pH24 above 6.0 is undesirable for human consumption because it leads to DFD (Dark Firm dry) meat. Dark Firm Dry meat is measured by L* coordinate which also depends on differences in pH24 (Wiklund et al., 2003; Rodríguez et al., 2011). The darker meat (low L* values) from Blackhead Persian may be attributed to reduced muscle glycogen and increased myoglobin content because of the light scattering properties of meat. The DFD meat is mostly due to transport stress and novelty of the environment.
The M. longissimus thoracis et. lumborum muscle from the SAMM breed was lighter as compared to DP and BP. The differences found between these breeds show that animals have different levels of susceptibility to stress especially the SAMM breed (Mota-Rojas et al., 2006). The higher L* value for SAMM is associated with the production of lactic acid prior to slaughter. Higher glycogen reserves credited other activities that occur post-mortem requiring energy such as breaking down the bond between muscle proteins to improve the tenderness of meat (Martinez-Cerezo et al., 2005). An increase in pH24 results in tougher meat as observed in SAMM. The WBSF values observed for BP could be attributed to the fact that this breed is a fat-tailed. Fat improves the tenderness of meat, and fat in meat contributes to the eating quality of mutton. There were no breed effects (p>0.05) on a* values. The reason could be that color of meat can also be affected by chemical stability of myoglobin in the muscle (Kannan et al., 2001; Jacob et al., 2005; Muchenje et al., 2009). Myoglobin stability is also affected by electrical stimulation, chilling light exposure, diet (Vitamin E supplementation) and sex (Rosenvold and Andersen, 2003) which were not measured in the current study. Significant breed effects were observed in CL and WBSF values. The highest (p<0.001) CL and (p<0.01) WBSF values were observed in meat from BP and SAMM, respectively. The meat from BP and DP was considered to be tender than that of SAMM. SA Mutton Merino had tougher meat and higher pH24. Increased tenderness could be due to the effects of pH24 activity on the proteolytic enzymes that degrade myofibrillar muscle structure (Wanatabe et al., 1996). In contrast, Miranda-de la Lama et al. (2009, 2011) reported increased tenderness of meat with higher pH24. However, Muchenje et al. (2008) indicated that higher pH24 may not always be involved in toughness of meat.
Correlations between the activity of CK and physico-chemical characteristics of mutton
Figure 1 shows the PCA for correlations between CK and physico-chemical characteristics of mutton. Creatine kinase was not related to any of the meat quality parameters. However, Warriss et al. (1990) and Mach et al. (2008) reported that CK can be linked to muscle exhaustion that occurs prior to slaughter. In the current study, results show only values after slaughter as it was conducted under practical conditions where no manipulations are made to the animals according to treatment. This causes muscle damage, increased CK activity and negatively affects the quality of meat (Yu et al., 2009). According to Wiklund et al. (2003), muscles of the animal in good physical condition contain enough glycogen to guarantee optimal pH24 values (5.5 to 5.7) in the meat. Depletion of glycogen is due to the extent of transport and lairage duration (Liu et al., 2012). Positive correlations were observed between physico-chemical characteristics; pH24, L*, a*, b*, CL% and WBSF. Higher pH24 found in the muscles was associated with darker meat (Miranda-de la Lama et al., 2009; Muchenje et al., 2009). The color of meat does not only depend on pH24 but on the type of muscle too (Jacob et al., 2007).

Principal component analyses for creatine kinase and physico-chemical characteristics of mutton. L* = Lightness, a* = Redness, b* = Yellowness, pHu = Meat Ph, Cook% = Cooking loss, CK = Creatine kinase, Thaw = Thawing loss percentage, WBSF = Warner Braztler Shear Force.
Meat pH24 affects other important meat quality attributes such as color, tenderness and water holding capacity (WHC) (Muchenje et al., 2008). According to Cloete et al. (2008), high pH24 has an effect on the color and tenderness of meat. It was also indicated that higher pH24 (>5.8) leads to undesirable beef color (Ferguson et al., 2001; Hoffman et al., 2003; Muchenje et al., 2009) and undesirable mutton color (Hopkins and Fogarty, 1998; Toohey and Hopkins; 2006) which is unattractive to the consumer (Vergara and Gallego, 2000; Esenbuga et al., 2009). Furthermore, the relationship between physico-chemical characteristics is also dependent on the differences in intramuscular fat, pH24, cooling rate, muscle fiber and myoglobin. Moisture content in the muscle, cooking temperatures and the amount of water lost during cooking have been found to be contributing factors towards physico-chemical characteristic (Combes et al., 2003; Kadim et al., 2008; Muchenje et al., 2008; Miranda-de la Lama et al., 2009). Martinez-Cerezo et al. (2005) indicated that the differences in the physico-chemical characteristics of mutton are due to the activities that occur in the muscle. However, the characteristics of mutton differ in their importance as shown by PCA analyses (Kadim et al., 2009).
In the current study, as shown in Table 5, CK contributed a greater proportion of variance as compared to the meat quality characteristics. This implies that there is no relationship between CK and physico-chemical characteristics of mutton. In lamb, about 49.67% was explained by PCA for the first four meat quality characteristics (Cańeque et al., 2004). Kumar and Singh (2010) used PCA to analyze the main parameters that were responsible for the main variability in water. They reported about 94.5% of the factors explaining the total variability. As explained earlier, the second, third and fourth components (pH24, L* and a*) were influenced by chemical compositions that include glycogen content in the muscle. However, it could not be determined which components did contribute to the indicated variances.
CONCLUSION
Meat quality and creatine kinase are negatively affected by pre-slaughter stress, season and breed. Summer affected the lightness, yellowness and tenderness of meat in increased the activity of CK. Meat for BP was tender than DP and SAMM with a decrease in pH24. Despite the fact that CK is a sensitive indicator of muscular damage and fatigue, it is not related to physico-chemical characteristics of mutton. Therefore, further studies are needed to identify the sources of variance for CK production and extent of muscular damage in relation to CK activity and physico-chemical characteristics of mutton during pre-slaughter stress under practical conditions. In addition, there is a need to assess other stress related enzymes produced during pre-slaughter stress and their effect on the quality of mutton.
Acknowledgements
The authors acknowledge the National Research Foundation (NRF, Project T079) of South Africa and Red Meat Research and Development Trust of South Africa (RMRDT-SA) for assisting in funding this research. Appreciation also goes to the municipal abattoir that allowed this study to be conducted on their premises.

