 |
 |
| Anim Biosci > Volume 34(8); 2021 > Article |
|
Abstract
Objective
Portable laser methane detectors (LMDs) may be an economical means of estimating CH4 emissions from ruminants. We validated an LMD-based approach and then used that approach to evaluate CH4 emissions from indigenous dairy cows in a dryland area of Ethiopia.
Methods
First, we validated our LMD-based approach in Simmental crossbred beef cattle (n = 2) housed in respiration chambers and fed either a high- or low-concentrate diet. From the results of the validation, we constructed an estimation equation to determine CH4 emissions from LMD CH4 concentrations. Next, we used our validated LMD approach to examine CH4 emissions in Fogera dairy cows grazed for 8 h/d (GG, n = 4), fed indoors on natural-grassland hay (CG1, n = 4), or fed indoors on Napier-grass (Pennisetum purpureum) hay (CG2, n = 4). All the cows were supplemented with concentrate feed.
Results
The exhaled CH4 concentrations measured by LMD were linearly correlated with the CH4 emissions determined by infrared-absorption-based gas analyzer (r2 = 0.55). The estimation equation used to determine CH4 emissions (y, mg/min) from LMD CH4 concentrations (x, ppm m) was y = 0.4259x+38.61. Daily CH4 emissions of Fogera cows estimated by using the equation did not differ among the three groups; however, a numerically greater milk yield was obtained from the CG2 cows than from the GG cows, suggesting that Napier-grass hay might be better than natural-grassland hay for indoor feeding. The CG1 cows had higher CH4 emissions per feed intake than the other groups, without significant increases in milk yield and body-weight gain, suggesting that natural-grassland hay cannot be recommended for indoor-fed cows.
According to the Food and Agriculture Organization of the United Nations, there are approximately 1.4 billion cattle worldwide [1], which together are a major source of greenhouse gas emissions. Indeed, 8% to 18% of greenhouse gas emissions (CO2 equivalent) due to anthropogenic activities are attributable to livestock farming [2]. In addition, more than 70% of the gastrointestinal methane (CH4, a major greenhouse gas) emissions in 2018 are attributed to cattle [3]. To reduce the greenhouse gas emissions associated with livestock farming, it will be important to develop methods of controlling the CH4 produced by fermentation processes in the gastrointestinal tracts of ruminants [4]. Such control will have the additional benefit of increasing livestock productivity through improved energy utilization.
In many developing countries, especially in dryland areas where cattle farming is often one of only a few viable livelihoods, cows are often grazed. However, overgrazing can result in serious soil erosion. Indeed, an estimated 73% of pasture and rangeland in the world’s drylands has been degraded, mostly as a result of overgrazing [3]. Ethiopia is one such country that is being impacted by serious soil erosion due to overgrazing; the rate of soil loss in Ethiopian rangelands (38.7 t/ha/yr) is more than five times higher than that in Ethiopian croplands (7.2 t/ha/yr) [5]. This greater soil loss in the rangelands is attributed to increased runoff resulting from intensive grazing and soil compaction [5]. To mitigate this soil loss, grazing is now restricted to areas that have little value for cropping, and indoor-fed animal production is being encouraged across the country.
To promote indoor feeding as an alternative to conventional grazing, accurate estimates of CH4 production from ruminants are necessary. For example, understanding enteric CH4 production by ruminants in different production systems is important for developing strategies to mitigate anthropogenic CH4 emissions [6]. Various methods for measuring CH4 have been developed. However, respiration chambers for open- or closed-circuit calorimetry, which are considered the gold standard for animal nutrition studies, are expensive to install and maintain [7]. The sulfur hexafluoride tracer gas technique is labor intensive and expensive with respect to changing the canisters worn around the animals’ backs and analyzing the collected samples [7]. A limitation of the GreenFeed system (C-Lock Inc., Rapid City, SD, USA) is that CH4 emissions cannot be measured unless the animals visit the monitoring station for feeding, and the frequency of visits may be affected by diet [8]. Therefore, cheaper and simpler methods of measuring CH4 with acceptable efficiency and precision are needed, especially in areas where it is financially challenging to obtain experimental equipment.
Portable laser methane detectors (LMDs) have been proposed as a potential economical means of estimating CH4 emissions without disturbing the normal activities of cattle. In this application, the device emits a laser beam that is directed at an animal’s nostril; the device then automatically measures the CH4 concentration (ppm m) along the length of the beam [9]. In a validation study, the CH4 concentrations in the exhaled air of 72 steers, as measured by LMD, were correlated with the concentrations measured by using respiration chambers (r2 = 0.39, p<0.01) [10]; in this study, the LMD values were obtained after first measuring the CH4 emissions in respiration chambers using the same animals. Another study reported the use of LMD on a farm to determine CH4 concentrations in the breath of 622 dairy cows; however, the values determined by LMD were not validated against another method [11]. Thus, further validation of the LMD approach by using values recorded simultaneously by means of an already validated method (e.g., respiration chambers) is needed before LMDs can be applied in feeding trials examining CH4 emissions.
Here, we examined the use of an LMD-based approach to estimate CH4 emissions through two in-vivo experiments for cattle. First, we validated our LMD-based approach against a respiration chamber-based approach in Simmental crossbred beef cattle (Exp 1). Then, we performed a feeding trial to examine the effects of indoor feeding on the CH4 emissions and lactation performance of Fogera dairy cows (Exp 2).
The cattle used in this study were treated according to the Tottori University provisions and regulations for animal experiments throughout all the experimental periods, under approval from the Committee of Animal Experiments of Tottori University (No. 20-T-17).
The CH4 emissions of cattle were estimated by both respiration chamber and LMD. Because of a lack of respiration chambers in Ethiopia (the site for Exp 2), we performed this experiment at Linze Grassland Agriculture Trial Station (39.24°N, 100.06°E), Lanzhou University, China, using two Simmental crossbred male beef cattle (not castrated; body weight [BW], 224 and 260 kg; age, 9 mo). The experimental period was 12 d (17 to 28 Sept 2019). Each animal was provided one of two diets throughout the experimental period: a high-concentrate diet (HC) comprising alfalfa hay (1.1 kg-dry matter [DM]/d), wheat straw (1.1 kg-DM/d), and commercial concentrate feed (1.5 kg-DM/d), or a low-concentrate diet (LC) comprising the same feed ingredients but at 2.5, 2.5, and 0.8 kg-DM/d, respectively (Supplementary Tables S1 and S2). Both diets were designed to provide the net energy and crude protein required for a bull to gain 1 kg BW daily on the basis of the estimation equation and tabular values of feed ingredients presented in Feeding Standard for Beef Cattle [12]. The daily DM intakes of roughage (alfalfa hay and wheat straw) and of the concentrate feed were recorded for each animal throughout the experimental period.
After 5 d in cubicle accommodation (i.e., on d 6 after the start of cubicle accommodation), each animal was transferred to an indirect open-circuit respiration calorimeter chamber (chamber capacity, 17.8 m3) for 7 d (4 d for adaptation and 3 d for measurement). The CH4 concentration in the exhaust air from each chamber was measured every 15 min for 48 h by using an infrared-absorption-based gas analyzer (VA-3000, Horiba Ltd., Kyoto, Japan). The air temperature and humidity in the chamber were recorded continuously and remained in the range from 12.2°C to 25.5°C and from 17.9% to 56.7%, respectively. Air influx in each chamber adjusted for the gas volume under standard conditions was recorded. On d 10, samples of the feed ingredients were collected to determine the concentrations of ash-free neutral detergent fiber (NDFom).
While the cattle were in the respiration chamber, CH4 concentrations were measured simultaneously by using both the gas analyzer and an LMD (SA3C32B, Tokyo Gas Engineering Co. Ltd., Tokyo, Japan) for two 12-h periods from 18:00 to 06:00. The LMD instrument uses a non-visible laser and infrared-absorption spectroscopy to measure the CH4 concentration (LMD-CH4) at 0.5-s intervals. The wavelength of the infrared ray is fixed at 1,653 nm, which corresponds to the absorption line of CH4.
LMD-CH4 was measured in the respiration chamber with the LMD held at a distance of 0.6 to 1.2 m from the animal’s nostrils. However, the frequent movement of cattle during the day made it difficult to accurately aim the LMD. A preliminary experiment prior to Exp 1 revealed that the average CH4 emissions (mg-CH4/min) of four cattle in the respiration chambers over two 23-h periods (each from 07:00 to 06:00) were highly correlated with the average values for the 12-h period from 18:00 to 06:00. The average CH4 emissions over 23 h (y, mg-CH4/min) were therefore estimated from those over 12 h (x, mg-CH4/min) with y = 1.072x–1.891 (r2 = 0.95). A similar correlation has been reported for eight steers in respiration chambers between 24-h and nocturnal (00:00 to 06:30) heat-production values (r2 = 0.81 to 0.90), and between 24-h heat-production and CH4 emissions (r2 = 0.55 to 0.66) [13]. Therefore, we assumed that the CH4 emissions measured at night would provide acceptable estimation of 24-h CH4 emissions.
LMD-CH4 was measured once an hour during the night for two 12-h periods (18:00 to 06:00) for each animal. Each of the LMD-CH4 measurement took less than 5 min, and 2 to 3 min of data per measurement were used after eliminating data not usable for analysis (e.g., data where the LMD was not pointing exactly at the nostril). Entry of a person into the chamber for the purpose of taking measurements was assumed to have minimal effects on the animals. Nevertheless, to reduce the effects of the person’s entry, we kept the doors of respiration chambers closed after the entry for each measurement, and standardized the length of time spent in the respiration chamber for each measurement.
In the preliminary LMD-CH4 datasets, two trends in the LMD-CH4 values were observed, one for eructation and another for respiration; this is consistent with a previous report [10]. Therefore, assuming a double normal distribution, each hourly LMD-CH4 dataset was split into two sub-datasets, one for eructation and one for respiration. A total of five statistical parameters were calculated for each dataset: the mean and the standard deviation for the LMD-CH4 values within each of the two sub-datasets and the ratio distribution for the two sub-datasets that achieved the highest likelihood. For the calculation of these five parameters, the nonlinear generalized reduced gradient solving (nonlinear GRG) method in Excel 2019 (Microsoft Corporation, Redmond, WA, USA) was used. The higher LMD measurements were assumed to represent CH4 emissions by eructation, whereas the lower LMD measurements were considered to represent CH4 emissions by respiration.
Then, the two probabilities for a single LMD-CH4 value, namely one in the normal distribution for respiration and the other in the normal distribution for eructation, were calculated. Each LMD-CH4 value was then categorized according to these probabilities into one of two sub-datasets (for eructation or for respiration) (Supplementary Figure S1). The LMD-CH4 datasets that could not be clearly separated into the two sub-datasets (i.e., dataset with a low power for the test for eructation and respiration) were excluded. Of the 42 LMD-CH4 datasets collected from the two cattle, 34 could be separated into two normal distributions, one each for respiration and eructation. The statistical power of the test for each of the 34 datasets ranged from 72.8% to 94.8%.
Each of the 34 datasets contained three mean values: ones for the two sub-datasets (for respiration and eructation) and the other for the combined sub-datasets (before their separation into respiration and eructation). Furthermore, three mean-value groups were obtained: the first group composed of 34 mean values for the 34 sub-datasets for respiration, the second group for the 34 sub-datasets for eructation, and the third group for the 34 datasets before separation into respiration and eructation. Each of the three mean-value groups was then regressed by using the least-squares method against the dataset obtained from the respiration chamber measurements.
During the analysis, we observed time delays for when the values obtained by the LMD were reflected in the values recorded by the gas analyzer. These delays were probably related to the distance from the respiration chamber to the gas analyzer, which was in the general control room. Therefore, we calculated correlation coefficients for each of the three mean-value groups and each of six datasets obtained with the gas analyzer at 0, 15, 30, 45, 60, and 75 min after the LMD-CH4 measurement. The correlation coefficients were calculated by using R statistical software (version 3.1.1, R Foundation for Statistical Computing, Vienna, Austria). By using the pair of datasets with the highest correlation coefficient, an equation to estimate daily CH4 emissions using the nocturnal LMD values was formulated.
A feeding trial for indigenous cows (Fogera breed) was performed for 24 d (from 21 Aug to 13 Sept 2019) at Andassa Livestock Research Center, Ethiopia (11.42 to 11.92°N, 37.07 to 37.65°E; elevation, 1,730 to 1,750 m above sea level). This center recently received 1,434 mm of annual rainfall, and the average daily temperature ranged from 8.8°C (in Jan) to 29.5°C (in Mar) (data supplied by the Andassa Research Center). Twelve multiparous (2 or 3 parity) dairy cows (mean BW, 227.4±23.1 kg) in midlactation (107±27 d in milk at the start of Exp 2) were allocated into one of three feeding groups: a grazing group (GG, n = 4; control) and two indoor-feeding groups fed with natural-grassland hay (CG1, n = 4) or with Napier-grass (Pennisetum purpureum) hay (CG2, n = 4).
The natural-grassland hay used as the feed for CG1 was purchased from a private dairy farm and was composed mainly of Andropogon, Cynodon, Digitaria, Hyparrhenia, and Panicum spp. as well as Trifolium quartinianum, Trifolium polystachyum, and Indigofera atriceps. In addition to these species, Trifolium subterraneum and Eleusine indica were observed on the grazing land of the research center used for GG. Napier grass was also examined because it was widely available and was assumed to be a major forage in the drylands of Ethiopia owing to its high DM yield (18 to 23 t-DM/ha/yr) [14] and high crude-protein content (15.8% DM) [15]. The Napier grass was harvested from irrigated land at the research center and air dried in the field for at least 3 d before use.
All three diets were designed to provide sufficient net energy and crude protein for a 3-kg daily milk yield by using the BW of the cows, the estimation equation presented in Nutrient Requirements of Dairy Cattle [16], and reported nutrient concentrations of the feed ingredients [17]. For the GG cows, natural-grassland hay, Napier grass, and concentrate were offered, respectively, at 0.0, 0.0, and 1.5 kg-DM/d; for the CG1 cows at 3.2, 0.0, and 1.5 kg-DM/d; and for the CG2 cows at 0.0, 3.8, and 1.5 kg-DM/d. The GG cows were expected to graze similar amounts of natural-grassland hay as the CG1 cows.
The daily feed allowance for each cow was adjusted on the basis of BW at the start of the experiment. Throughout the experimental period, the GG cows were allowed to graze daily from 8:00 to 16:00 and were accommodated indoors during rest hours; no roughage (natural-grassland hay or Napier grass) was provided when the cows were accommodated indoors. The CG1 and CG2 cows were provided with natural-grassland hay and Napier grass, respectively, twice a day (at 08:00 and 17:00). The roughage for CG1 and CG2 was chopped into 5- to 10-cm lengths for feeding. The feed for all the groups was supplemented with concentrate feed when the cows were milked twice a day at 07:00 and 16:00. The concentrate consisted (on a DM basis) of maize grain (40%), Noug seed cake (49%), wheat bran (8%), salt (1%), and ruminant premix (2%; Intraco Ltd., Antwerp, Belgium). All the cows were offered water twice a day during the daytime.
As described for Exp 1, LMD-CH4 values were recorded for each cow each hour for 2 nights (i.e., two periods of 18:00 to 06:00) after the adaptation period had passed (from d 6). Of the 286 datasets of hourly LMD-CH4 measurements from the 12 cattle, 263 could be separated into two normal distributions for respiration and eructation. The statistical power of the test for eructation and respiration in each of the 263 datasets ranged from 75.3% to 98.1%. By using the regression equation obtained in Exp 1, the mean value of each of the three mean-value groups—for eructation, respiration, or both—was converted into a daily CH4 emission for each cow.
The weight of feed offered and refusals were recorded daily throughout the experimental period to calculate daily feed intake. Samples of the feed ingredients (grazing herbage, natural-grassland hay, Napier grass, and concentrate) were collected for chemical analysis on d 17. The BW of each cow was recorded at the start and end of the experiment, and on the days of LMD measurement. Daily milk yields (summation of both the morning milking and afternoon milking) were measured throughout the experimental period.
To examine the fecal excretions and determine digestive coefficients for all of the cows, spot fecal samples (about 500 g/sample) were collected three times a day from d 17 to 21 and stored at −15°C until analysis. In addition, to estimate the DM intake for the four GG cows, 2.5 g of ground chromium oxide (Cr2O3) was mixed with the concentrate feed provided twice a day, from d 12 to 18, and again spot samples fecal were collected.
The feed and fecal samples were dried at 105°C in a forced-air oven for more than 6 hours to constant weight and ground to pass through a 1-mm screen. Then, by using the standard methods of the Association of Official Analytical Chemists [18], the concentrations of crude protein (method no. 984.13), ether-extracted fat (crude fat; 920.39), ash-free acid detergent fiber and acid detergent lignin (973.18), and crude ash (942.05) in the dried feed and fecal samples were determined. The concentration of NDFom was determined as reported elsewhere [19]. Fecal Cr2O3 concentrations were also determined as reported elsewhere [20], and the weight of fecal excretions of the GG cows were estimated. The DM digestive coefficients of all the cows were then calculated by using the acid detergent lignin concentrations in the feed and fecal samples as internal markers. We used the DM digestive coefficients and the weight of fecal excretions to calculate the DM intake of GG cows.
Two estimates of CH4 emissions were calculated by using the following equations reported by Niu et al [21] and Hristov et al [22], respectively:
The GEI value used in the equation of Hristov et al [22] was calculated by using an equation reported elsewhere [23]. These two estimates were compared with the CH4 emissions recorded by the gas analyzer in Exp 1 and with those estimated by using the LMD in Exp 2.
Each of the datasets obtained in Exp 2 was analyzed by using the model yij = μ+αi+ɛij, where yij is the dependent variable, μ is the overall mean value for each dataset, αi is the fixed effect of treatments (feeding style and ingredients), and ɛij is the random residual error of the jth cow with the ith treatment. Differences in means among the three groups were tested by using one-way analysis of variance. When the treatment effect was significant (p<0.05), multiple comparisons were tested using Tukey’s method. These statistical analyses were performed with R statistical software (version 3.1.1, R Foundation for Statistical Computing, Vienna, Austria).
For both cattle, the diets were almost all consumed shortly after the start of feeding. The daily intake of feed and nutrients, and the ratio of concentrate-feed intake to total DM intake are shown in Table 1. The amounts of NDFom in the alfalfa hay, wheat straw, and concentrate feed were 52.7%, 77.2%, and 22.8%-DM, respectively. Gas analysis revealed that CH4 emissions increased immediately after feeding (Figure 1). The CH4 emissions (mg/kg0.75 BW/15-min) at the three feeding times between the two nocturnal LMD-measuring periods were 17.3, 14.4, and 29.9 for the cattle fed HC, and 11.7, 16.1, and 19.2 for the cattle fed LC. In addition, the peak CH4 emissions after each of the three feedings were 27.2, 36.2, and 41.1 for the cattle fed HC, and 21.8, 20.2, and 26.3 for the cattle fed LC. The average daily CH4 emission was 1.91 and 1.53 g/kg BW0.75 for the cattle fed the HC and LC diets, respectively (Table 1). The CH4 emissions (mg/kg0.75 BW/15-min) of the cattle fed the HC diet were higher than those of the cattle fed the LC diet at 123 of the total of 145 datapoints (Figure 1).
The mean-value group comprising the respiration sub-datasets (x, ppm m) was most significantly correlated with the gas analyzer dataset up to 60 min after the LMD-CH4 measurement (y, mg-CH4/min; Table 2). Using these two datasets, the regression equation was y = 0.4259x+38.61 (r2 = 0.55, p<0.001, Figure 2).
The NDFom concentration in the Napier grass used in the CG2 diet was lower than that in the natural-grassland hay used in the CG1 diet and that of the grazing grasses (Table 3); therefore, the NDFom concentration of ingested feed was lower in CG2 than in CG1 and GG (Table 4). The crude-protein concentration in the Napier grass was higher than that in the natural-grassland hay and the grazing grasses (Table 3), and the crude-protein intake in CG2 was the highest among the three groups (Table 4). The calculated GEI was 21.1, 18.5, and 22.0 Mcal/d for GG, CG1, and CG2, respectively. DM intake was comparable between GG and CG2 (p = 0.13), and GG and CG1 (p = 0.09), but it was significantly lower in CG1 than in CG2 (p<0.01, Table 4). NDFom intake was lower in CG1 than in GG (p<0.01), whereas the ratio of concentrate intake to total DM intake was higher in CG1 (p = 0.01) and lower in CG2 (p = 0.05) than in GG. DM digestibility was lower in CG1 than in GG (p<0.01), whereas NDFom digestibility was lower in CG1 and CG2 than in GG (both p<0.01). Body-weight gain was higher in CG2 than in CG1 (p = 0.03) and was negative in CG1. The estimated CH4 emissions (g/kg0.75 BW/d) did not differ among the three groups (Table 4). CH4 emissions per milk yield did not differ among the groups (p = 0.95). However, CH4 emissions per DM intake and the ratio of CH4 emissions to estimated GEI were significantly higher in CG1 than in the other groups (both p<0.05).
In Exp 1, prompt increases in CH4 emissions after feeding were detected by the gas analyzer (HC 17.3–29.9 to 27.2–41.2, LC 11.7–19.2 to 20.2–26.3 mg/kg BW0.75/15-min; Figure 1) and were consistent with previously reported values for CH4 emissions during feeding (15 to 50 mg/kg0.75 BW/15-min) [24].
More than 80% of the hourly measurement datasets could be used to produce the two normal distributions, indicating that the measurement duration (<5 min) used here, which was similar to that used in previous studies (4 min [10], <5 min [25]), was appropriate. A previous study reported that the total time spent in eructation as a percentage of the total LMD-measurement time ranged from 28.7% to 49.4% [10]. In our study, the percentage of LMD-CH4 values categorized into eructation in each of the LMD-CH4 datasets—which could be regarded as the percentage of total time spent in eructation—ranged from 11.7% to 48.3% (Exp 1). Our results thus appeared to be consistent with those of the previous study [10]. Each LMD-CH4 value in the LMD-CH4 dataset could be properly categorized into one of the two sub-datasets for eructation and respiration.
For all the periods (after LMD measurement) used for the regression, the respiration sub-datasets were correlated more significantly than the eructation datasets with the CH4 emissions dataset determined with the gas analyzer (Table 2). Although more than 80% of the CH4 exhaled by cattle is associated with eructation [10], the higher correlation coefficients obtained here by using the respiration sub-datasets indicated that respiration was more useful than eructation for quantifying the CH4 emissions of individual cattle. Moreover, the respiration sub-datasets were well correlated with the gas-analyzer dataset for 0 to 60 min after LMD-CH4 measurement (Table 2, Figure 2). The time delay until when the values obtained by the LMD were reflected in the values recorded by the gas analyzer was thus estimated as 60 min. The correlation coefficient (up to 60 min, r2 = 0.55) was higher with this dataset than it was with the datasets obtained for the final 45 min (15 to 60 min, r2 = 0.46), 30 min (30 to 60 min, r2 = 0.46), and 15 min (45 to 60 min, r2 = 0.42) periods after LMD measurement. The decrease in r2 value with increasing time after LMD measurement suggested that diffusion of CH4 exhaled by the animal started affecting the values recorded by the gas analyzer immediately after the LMD-CH4 measurement.
A previous study [10] reported three equations with high goodness of fit for the estimation of CH4 emissions, using three statistical parameters obtained from each LMD dataset: the ratio of total time spent in eructation to total measurement time, the maximum CH4 concentration (ppm m) during the total time spent in respiration, or a combination of both parameters. We calculated correlation coefficients for each of these three parameters for the CH4 emissions dataset obtained for 60 min (0 to 60 min) after LMD-CH4 measurement. However, all three correlation coefficients calculated (r2<0.01, = 0.07, and = 0.44, respectively) were lower than the coefficients demonstrated in our study (r2 = 0.55).
Ultimately, we constructed an equation to estimate the CH4 emissions in Exp 2 on the basis of the CH4 emissions for 0 to 60 min after LMD-CH4 measurement and the LMD-CH4 respiration dataset. The resulting estimation equation was y = 0.4259x+38.61 (y, CH4 concentration [mg/min]; x, mean of respiration sub-datasets recorded by LMD [ppm m]).
Previously, at the site of Exp 2, the crude-protein concentration (on a DM basis) in Napier grass was reported as 9.3% [17]. In our study, the crude-protein concentration in the Napier grass was 8.2% (Table 3). In these two studies, the agronomic practices for Napier grass production followed those previously recommended for this grass (International Livestock Research Institute accession number 15743) [26]. A crude-protein concentration of at least 8% (on a DM basis) is needed if forage is given as a sole diet to ruminants [27]. The higher Napier grass crude-protein concentrations in both our study and the former study (9.3% [17]) than in the latter study (8% [27]) indicated that this grass could be used as a basal diet for Fogera dairy cows. By contrast, the crude-protein concentration in natural-grassland hay in our study (4.5%; Table 3) was lower than that in the Napier grass (8.2%). The concentrations of the crude protein and NDFom (72.1%) in the natural-grassland hay were consistent with those in the previous study (crude protein, 4.2%; NDFom, 74.2% [17]). These findings appeared to suggest that natural-grassland hay could not be used as a basal diet for the Fogera dairy cows, and that a concentrate supplement would be needed if natural-grassland hay were fed as the basal forage.
The similarity in the CH4 emissions per metabolic body size and in the ratios of emitted-CH4 energy to GEI observed between the cows in GG and CG2, and the numerically (albeit not significantly) higher milk yield and BW gain in CG2 than in GG (Table 4) suggested that Napier grass was a suitable forage for indoor feeding. The CH4 emissions per milk yield in CG2 and GG were also comparable (46.8 g/L-milk vs 49.3 g/L-milk; p = 0.97).
The concentration of dietary fiber (i.e., NDFom) affects voluntary feed intake through physical regulation via rumen fill [28]. Less fibrous diets with low NDFom concentrations promote dietary passage through the rumen and increase DM intake and decrease digestibility [28]. This may explain the slight increase in DM intake but decrease in DM digestibility in CG2 compared with in GG (Table 4). Likewise, the significantly lower DM digestibility in CG1 than in GG (p<0.01) was accompanied by a significantly lower dietary NDFom concentration and a significantly higher ratio of concentrate intake to total DM intake (both p<0.05). The lack of a significant difference in DM intake between CG1 and GG (p = 0.09) and the slightly higher CH4 emissions in CG1 than in GG (p = 0.20) resulted in significantly higher CH4 emissions per DM intake in CG1 (p<0.01). Nevertheless, milk yield (L/d) did not differ between GG and CG1 despite the lower DM digestibility in CG1 than in GG.
Daily BW gain was insignificant in CG1 but positive in the other groups. The metabolizable energy required for maintenance of indoor-fed cows of 600-kg BW is 7% less than that of cows grazing for 2 h/d [16]. The GEI decrease in CG1 compared with GG was 12.5%, although the DM intake did not significantly differ between CG1 and GG (p = 0.09). The GEI decrease in CG1 was more than the difference between the energy intakes required for grazing and for indoor-feeding. By contrast, the milk yield was comparable between CG1 and GG (p = 0.81). Milk yields in all three groups were less than 3 L/d (the milk-yield target). The GEI decrease in CG1 compared with GG—which was more than the decrease acceptable for BW gain—and the comparable milk yield (albeit both less than the target yield) between CG1 and GG might have led to the lack of BW gain in CG1. Together with the significantly lower DM digestibility in CG1 than in GG, these findings suggest that natural-grassland hay is not a suitable feed for indoor feeding. The lower CH4 emissions per DM intake in CG2 than in CG1 were consistent with the findings of a previous report [17], and with those of another report that demonstrated that a less fibrous diet with a low NDFom concentration (i.e., CG2) decreases CH4 emissions [29].
The high statistical powers of the test used to separate the LMD-CH4 dataset into eructation and respiration sub-datasets in both experiments, and the fact that the respiration sub-datasets were moderately well correlated with the CH4 emissions dataset collected by the gas analyzer in Exp 1 (Figure 2), indicated that the maximum likelihood estimation was appropriate for obtaining a group of datasets that could be used to provide an equation to estimate CH4 emissions.
The CH4 emissions per metabolic body size estimated in Exp 1 (Table 1) and Exp 2 (Table 4) were lower than those calculated by using the equations of Niu et al [21] (HC [Exp 1], 2.64; LC [Exp 1], 2.98; GG [Exp 2], 3.15; CG1 [Exp 2], 3.10; CG2 [Exp 2], 3.22 g/kg0.75 BW/d) and Hristov et al [22] (HC [Exp 1], 2.33; LC [Exp 1], 3.37; GG [Exp 2], 2.93; CG1 [Exp 2], 2.70; CG2 [Exp 2], 2.82 g/kg0.75 BW/d). These gaps could not be eliminated by recalculation of the CH4 emissions by using the correlation between those for 23 h (y) and for nocturnal 12 h (x) as revealed in our preliminary experiment (y = 1.072x–1.891). Nevertheless, the ratios of CH4 emissions to estimated GEI in Exp 2 (4.1% to 5.0%) were consistent with previously reported ratios (2% to 15% [30]). The NDFom concentrations of the ingested diets in Exp 1 (LC, 59%; HC, 48%) and Exp 2 (GG, 65.5%; CG1, 60.4%; CG2, 59.3%) were higher than those used by Niu et al [21] and Hristov et al [22] for constructing their equations (35.4%±7.66% and 34.3%± 7.47%, respectively). In contrast, DM intake in both of our experiments (Exp 1, 3.7 to 5.6 kg-DM/d; Exp 2, 4.20 to 4.93 kg-DM/d) was lower than those used by Niu et al [21] and Hristov et al [22] for constructing their equations (18.5±4.60 kg-DM/d and 16.5±4.30 kg-DM/d, respectively). The lower CH4 emissions that we obtained, despite the use of high fiber diets in all of the groups, might have been due to the inappropriate extrapolation of values by the previously reported equations, which were constructed by using datasets of cattle breeds different from those used here (Holstein, Ayrshire, Jersey, Brown Swiss [21]; Holstein, Jersey, Angus, Hereford [22]).
Here, we report several findings. First, the equation that we obtained to estimate CH4 emissions (y, mg/min) from LMD CH4 concentrations (x, ppm m) was y = 0.4259x+38.61 (r2 = 0.55). We also observed no difference in the CH4 emissions in CG2 compared with in GG, suggesting that Napier grass is a suitable feed for indoor feeding, and this finding was supported by the preferable milk yield in CG2. Moreover, we demonstrated that LMDs can be used to test feeding regimens with consideration of the milk productivity and CH4 emissions of dairy cows. Feeding regimens to both increase productivity and reduce greenhouse-gas emissions (i.e., to improve energy utilization) are important, especially in areas under financial constraints to feed cows with commercial concentrate feeds. The use of LMDs will make conducting feeding trials cheaper and simpler than using the other methods currently available for determining CH4 concentrations. This will be useful for studies conducted in such financially challenged areas, particularly in developing countries.
ACKNOWLEDGMENTS
We acknowledge the support of the Andassa Livestock Research Center, Amhara Region Agricultural Research Institute, Ethiopia, and the College of Grassland Science of Lanzhou University, China, for providing the respiration chambers (registered as LZUCKY-S-DXCLZ-001 for Institute of Grassland and Livestock Production System, Lanzhou University). This study was supported by the Marginal Region Agriculture Project of Tottori University; the Science and Technology Research Partnership for Sustainable Development (SATREPS) Project for Development of Next-generation Sustainable Land Management (SLM) Framework to Combat Desertification (JPMJSA1601) of the Japan Science and Technology Agency and Japan International Cooperation Agency; the Strategic Priority Research Program of Chinese Academy of Science (XDA20100102); the Key R & D Program of Ningxia Hui Autonomous Region (2019BBF02001); and the Program for Changjiang Scholars and Innovative Research Team at the University of China (IRT17R50).
Figure 1
Methane emissions of Simmental crossbred beef cattle in respiration chambers, as determined by infrared-absorption-based gas analyzer (Exp 1). □, cow (body weight, 224 kg) fed high-concentrate diet; ■, cow (body weight, 260 kg) fed low-concentrate diet; ●, feeding time. Methane emissions increased immediately after feeding.

Figure 2
Linear regression of methane concentrations recorded by laser methane detector (LMD, x) versus average methane emissions recorded by infrared-absorption-based gas analyzer for 60 min after LMD measurement (y) in Exp 1. For both measurements, the cattle (Simmental beef cattle) were held in respiration chambers. LMD-recorded methane concentration (x) of each datapoint was the mean value of methane concentrations, which were considered to represent methane emissions by respiration in each hourly measurement.
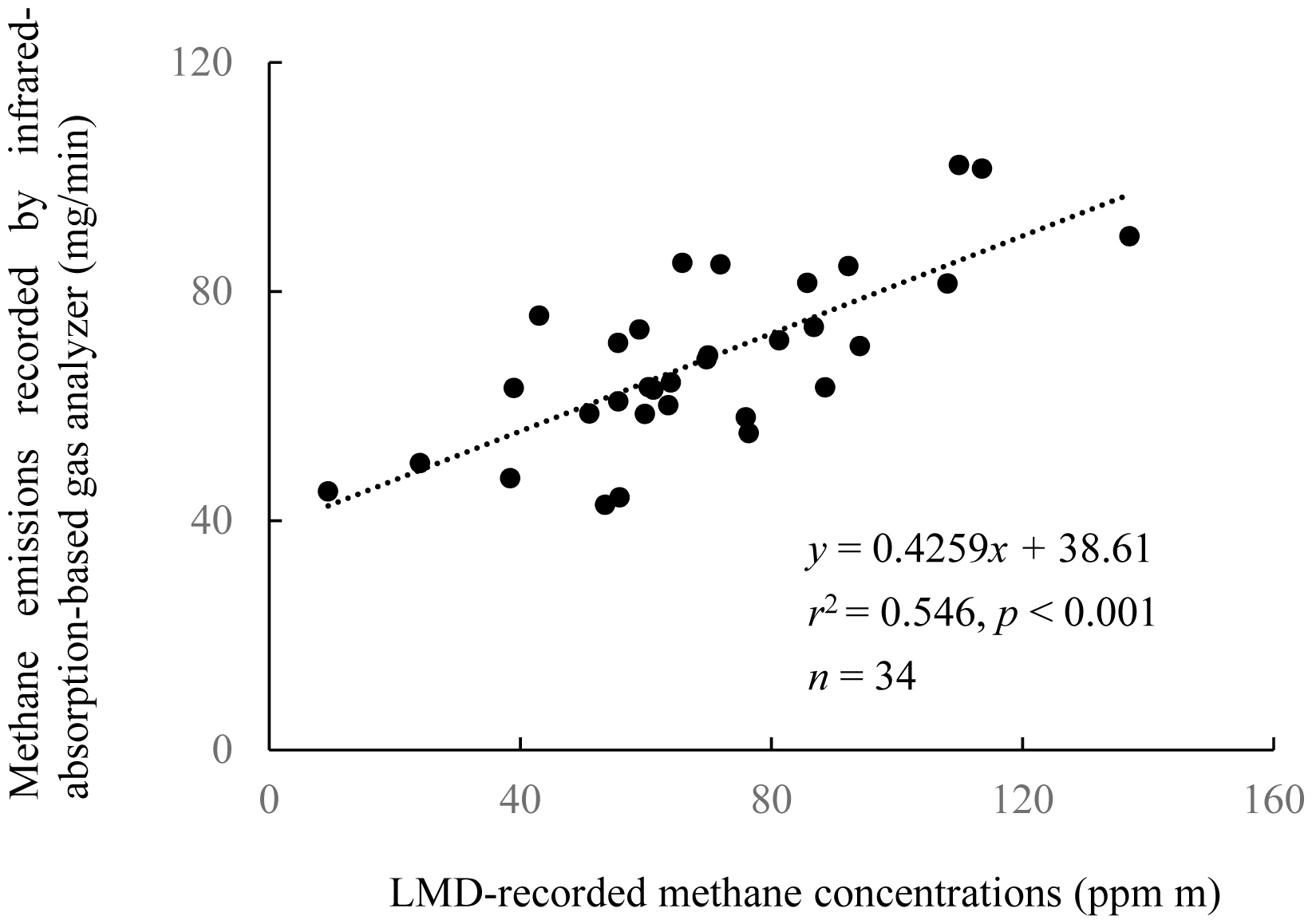
Table 1
Feed and nutrient intake, methane emissions, and daily body-weight gain of two Simmental crossbred beef cattle fed a high- or low-concentrate diet (Exp 1)
Table 2
Correlation coefficients between the infrared-absorption-based gas analyzer and laser methane detector datasets in Exp 1
| Items | Respiration | Eructation | Overall |
|---|---|---|---|
| Period used for regression after the LMD measurement, min | |||
| 0 | 0.1957** | 0.1158 | 0.1213* |
| 0 to 15 | 0.2250** | 0.1767* | 0.1759* |
| 0 to 30 | 0.3401*** | 0.1225* | 0.1709* |
| 0 to 45 | 0.4817*** | 0.2254** | 0.2999** |
| 0 to 60 | 0.5463*** | 0.2224** | 0.3038** |
| 0 to 75 | 0.4532*** | 0.1663* | 0.2216** |
Table 3
Chemical compositions of the feed ingredients used in Exp 2
Table 4
Feed and nutrient intake, digestibility, milk yield, methane emissions, and body-weight gain in Fogera dairy cows (Exp 2)
| Items | GG1) | CG11) | CG21) | SEM | p-value |
|---|---|---|---|---|---|
| Feed and nutrient intake | |||||
| DM (kg/d) | 4.59ab | 4.20b | 4.93a | 0.226 | 0.004 |
| Crude protein (kg/d) | 0.33c | 0.38b | 0.55a | 0.024 | <0.0005 |
| Crude-protein concentration (%) | 7.21c | 9.04b | 11.1a | 0.148 | <0.0005 |
| NDFom (kg/d) | 3.00a | 2.54b | 2.93a | 0.137 | 0.002 |
| NDFom concentration (%) | 65.5a | 60.4b | 59.3c | 0.409 | <0.0005 |
| Ratio of concentrate intake to total DM intake (%) | 28.1b | 30.7a | 26.2c | 0.992 | <0.0005 |
| Digestibility | |||||
| DM (%) | 58.6a | 46.4b | 50.8ab | 3.990 | 0.006 |
| NDFom (%) | 72.8a | 54.8b | 61.3b | 4.292 | 0.0007 |
| Milk yield (L/d) | 1.33 | 1.56 | 1.64 | 0.478 | 0.70 |
| Milk yield (L/kg-DM intake/d) | 0.29 | 0.37 | 0.33 | 0.103 | 0.61 |
| Methane emissions | |||||
| g/d | 65.9 | 69.5 | 66.1 | 2.927 | 0.17 |
| g/kg0.75 BW/d | 1.12 | 1.20 | 1.12 | 0.092 | 0.44 |
| Methane-energy/GEI (%) | 4.14b | 5.00a | 3.99b | 0.205 | <0.0005 |
| Body-weight gain (kg/d) | 0.25ab | −0.07b | 0.55a | 0.273 | 0.03 |
Data for milk yield of one cow in GG could not be collected; therefore, the available data were used (n = 3).
SEM, standard error of means; DM, dry matter; NDFom, ash-free neutral detergent fiber; BW, body weight; GEI, gross-energy intake.
REFERENCES
1. FAO (Food and Agriculture Organization of the United Nations). FAOSTAT [Internet]. Statistics Division, FAO; 2020. [cited 2020 Jul 1]. Available from: http://www.fao.org/faostat/en/#data/QA
2. O’Mara FP. The significance of livestock as a contributor to global greenhouse gas emissions today and in the near future. Anim Feed Sci Technol 2011; 166–7:7–15.
https://doi.org/10.1016/j.anifeedsci.2011.04.074

3. FAO (Food and Agriculture Organization of the United Nations). FAOSTAT [Internet]. Statistics Division, FAO; 2020. [cited 2020 Nov 12]. Available from: http://www.fao.org/faostat/en/#data/GE/visualize
4. Gerber PJ, Hristov AN, Henderson B, et al. Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: a review. Animal 2013; 7:Suppl 2220–34.
https://doi.org/10.1017/S1751731113000876


5. Haregeweyn N, Tsunekawa A, Nyssen J, et al. Soil erosion and conservation in Ethiopia: a review. Prog Phys Geogr 2015; 39:750–74.
https://doi.org/10.1177/0309133315598725

6. Weiske A, Vabitsch A, Olesen JE, et al. Mitigation of greenhouse gas emissions in European conventional and organic dairy farming. Agric Ecosyst Environ 2006; 112:221–32.
https://doi.org/10.1016/j.agee.2005.08.023

7. Garnsworthy PC, Difford GF, Bell MJ, et al. Comparison of methods to measure methane for use in genetic evaluation of dairy cattle. Animals 2019; 9:837
https://doi.org/10.3390/ani9100837



8. Hammond KJ, Jones A, Humphries DJ, Crompton LA, Reynolds CK. Effects of diet forage source and neutral detergent fiber content on milk production of dairy cattle and methane emissions determined using GreenFeed and respiration chamber techniques. J Dairy Sci 2016; 99:7904–17.
https://doi.org/10.3168/jds.2015-10759


9. Chagunda MGG, Ross D, Roberts DJ. On the use of a laser methane detector in dairy cows. Comput Electron Agric 2009; 68:157–60.
https://doi.org/10.1016/j.compag.2009.05.008

10. Ricci P, Chagunda MGG, Rooke J, et al. Evaluation of the laser methane detector to estimate methane emissions from ewes and steers. J Anim Sci 2014; 92:5239–50.
https://doi.org/10.2527/jas.2014-7676


11. Mühlbach S, Dorg D, Rosner F, Kecman J, Swalve HH. Genetic analyses for CH4 concentrations in the breath of dairy cows measured on-farm with the laser methane detector. In : Proceedings of the World Congress on Genetics Applied to Livestock Production; 2018 Feb 11–16; Auckland, New Zealand.
12. MOA (Ministry of Agriculture of the People’s Republic of China). Feeding Standard for Beef Cattle [Internet]. MOA: Beijing; 2004. [cited 2015 Jul 18]. Available from: http://wenku.baidu.com/view/a112b1a1c77da26925c5b0f1.html(In Chinese)
13. Derno M, Jentsch W, Schweigel M, Kuhla S, Metges CC, Matthes HD. Measurements of heat production for estimation of maintenance energy requirements of Hereford steers. J Anim Sci 2005; 83:2590–7.
https://doi.org/10.2527/2005.83112590x


14. International Livestock Research Institute (ILRI). Forages for the Future [Internet]. Nairobi, Kenya: ILRI; 2018. [cited 2020 Jun 10]. Available from: https://n2africa.org/sites/default/files/inline-images/Forages%20Newsletter_No7_fin.pdf
15. Rambau MD, Fushai F, Baloyi JJ. Productivity, chemical composition and ruminal degradability of irrigated Napier grass leaves harvested at three stages of maturity. S Afr J Anim Sci 2016; 46:398–408.
https://doi.org/10.4314/sajas.v46i4.8

16. NRC (National Research Council). Nutrient requirements of dairy cattle. 7th revised edWashington, DC, USA: The National Academy Press; 2001.
17. Mekuriaw S, Tsunekawa A, Ichinohe T, et al. Effect of feeding improved grass hays and Eragrostis tef straw silage on milk yield, nitrogen utilization, and methane emission of lactating fogera dairy cows in Ethiopia. Animals 2020; 10:1021
https://doi.org/10.3390/ani10061021



18. AOAC (Association of Official Analytical Chemists). Official method of analysis. 16th edArlington, VA, USA: Association of Official Analytical Chemists (AOAC); 1995. p. 19–20. Chapter 4.
19. Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Daily Sci 1991; 74:3583–97.
https://doi.org/10.3168/jds.S0022-0302(91)78551-2

20. Takemasa M. Improvement of the method for chromic oxide determination with potassium phosphate reagent. Bull Nat Inst Anim Ind 1992; 52:7–13. (In Japanese)
21. Niu M, Kebreab E, Hristov AN, et al. Prediction of enteric methane production, yield, and intensity in dairy cattle using an intercontinental database. Glob Chang Biol 2018; 24:3368–89.
https://doi.org/10.1111/gcb.14094



22. Hristov AN, Oh J, Firkins JL, et al. Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J Anim Sci 2013; 91:5045–69.
https://doi.org/10.2527/jas.2013-6583


23. NARO (National Agricultural and Food Research Organization). Standard Tables of Feed Composition in Japan 2009. Tokyo, Japan: Japan Livestock Industry Association; 2010. (In Japanese)
24. Du W, Hou F, Tsunekawa A, Kobayashi N, Ichinohe T, Peng F. Effects of the diet inclusion of common vetch hay versus alfalfa hay on the body weight gain, nitrogen utilization efficiency, energy balance, and enteric methane emissions of crossbred Simmental cattle. Animals 2019; 9:983
https://doi.org/10.3390/ani9110983



25. Chagunda MGG, Ross D, Rooke J, et al. Measurement of enteric methane from ruminants using a hand-held laser methane detector. Acta Agric Scand A Anim Sci 2013; 63:68–75.
https://doi.org/10.1080/09064702.2013.797487

26. Wondimeneh M, Biadegelegn H, Meseganaw W, Tekaba E, Mekonnen T, Adebabay K. Selection of productive napier grass varieties (Pennisetum Purpureum) at Andassa Livestock Research Center, North West Ethiopia. In : Proceedings of the 9th Annual Regional Conference on Completed Livestock Research Activities; 2016 Mar 9–20; Bahir Dar, Ethiopia. Amhara Regional Agricultural Research Institute; 2016.
27. Minson DJ. Nutritional differences between tropical and temperate pastures. Morley FHW, editorGrazing animals. Farunham Royal, Slough, UK: Commonwealth Agricultural Bureaux; 1980. p. 167–82.
28. Ichinohe T, Tamura T, Ueda K, Okubo M, Asahida Y. The particle size distributions of ingested boli, rumen digesta and feces in sheep fed orchardgrass hay harvested at different stages of maturity. Anim Sci Technol 1994; 65:701–8.
https://doi.org/10.2508/chikusan.65.701

29. Dong L, Li B, Diao Q. Effects of dietary forage proportion on feed intake, growth performance, nutrient digestibility, and enteric methane emissions of Holstein heifers at various growth stages. Animals 2019; 9:725
https://doi.org/10.3390/ani9100725



30. Flachowsky G, Lebzien P. Effects of phytogenic substances on rumen fermentation and methane emissions: a proposal for a research process. Anim Feed Sci Technol 2012; 176:70–7.
https://doi.org/10.1016/j.anifeedsci.2012.07.009

- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print





