 |
 |
| Anim Biosci > Volume 33(5); 2020 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
ACKNOWLEDGEMENTS
Figure 1

Figure 2
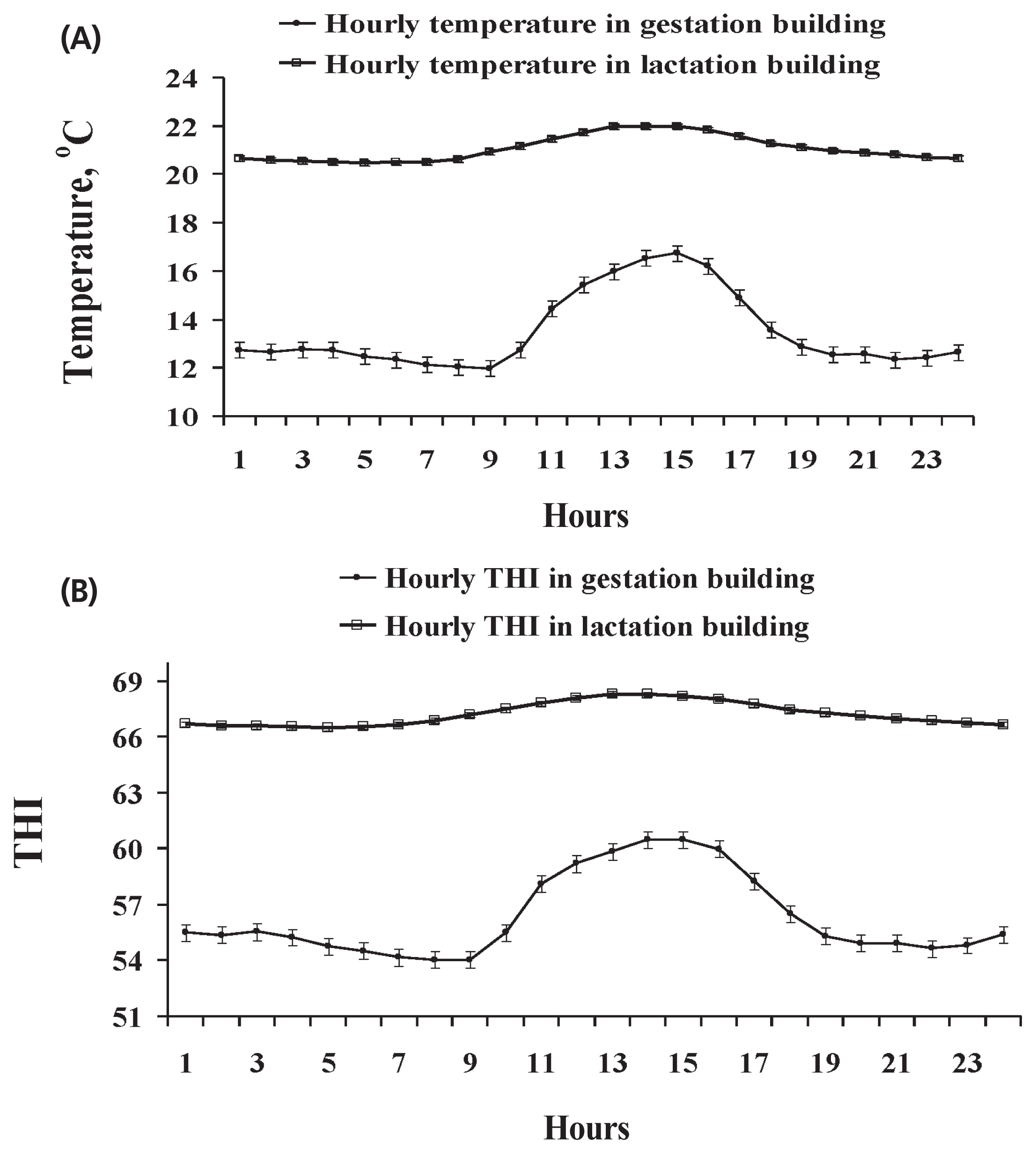
Table 1
| Item | Gestation | Lactation |
|---|---|---|
| Ingredient | ||
| Corn, yellow (%) | 81.30 | 74.00 |
| Soybean meal, 48% CP (%) | 13.85 | 19.60 |
| Poultry fat (%) | 1.00 | 3.99 |
| L-Lys (%) | 0.00 | 0.25 |
| L-Thr (%) | 0.00 | 0.01 |
| Limestone (%) | 1.11 | 1.08 |
| Dicalcium phosphate (%) | 2.05 | 2.38 |
| Salt (%) | 0.50 | 0.50 |
| Trace mineral permix1) (%) | 0.15 | 0.15 |
| Vitamin permix2) (%) | 0.04 | 0.04 |
| Total | 100.00 | 100.00 |
| Calculated composition | ||
| DM (%) | 89.7 | 90.1 |
| ME (Mcal/kg) | 3.3 | 3.5 |
| CP (%) | 13.3 | 15.8 |
| Lys (%) | 0.63 | 0.92 |
| Met (%) | 0.49 | 0.51 |
| Trp (%) | 0.14 | 0.17 |
| Thr (%) | 0.49 | 0.55 |
| Ca (%) | 1.03 | 1.12 |
| Total P (%) | 0.69 | 0.76 |
1) The trace mineral premix provided per kilogram of complete diet: 3.96 mg of Mn as manganous oxide; 16.5 mg of Fe as ferrous sulfate; 16.5 mg of Zn as zinc sulfate; 1.65 mg of Cu as copper sulfate; 0.30 mg of I as ethylenediamine dihydroiodide; and 0.30 mg of Se as sodium selenite.
2) The vitamin premix provided per kilogram of complete diet: 8,228 IU of vitamin A as vitamin A acetate; 1,173 IU of vitamin D3; 47 IU of vitamin E; 0.03 mg of vitamin B12; 5.88 mg of riboflavin; 23.52 mg of D-pantothenic acid as calcium panthonate; 35.27 mg of niacin; 0.24 mg of biotin; 1.76 mg folic acid; 3.88 mg menadione.
Table 2
| Item | Gestation | Lactation | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| d 352) | d 60 | d 90 | d 109 | d 1 | d 18 | |||
| Exp. 1 | ||||||||
| n3) | 11 | 11 | 11 | 11 | 11 | 11 | ||
| Malondialdehyde (μM) | 6.14 | 6.39AB | 7.51AB | 8.54A | 8.39AB | 6.02B | 1.05 | 0.096 |
| Protein carbonyl (nmol/mg) | 1.41 | 1.00a | 1.06a | 2.29c | 1.40ab | 1.76b | 0.13 | <0.001 |
| 8-hydroxy-deoxyguanosine (ng/mL) | 0.32 | 0.59ab | 0.61ab | 0.95b | 0.62ab | 0.29a | 0.12 | 0.006 |
| Exp. 2 | ||||||||
| n3) | 12 | 12 | 12 | 12 | 12 | 12 | ||
| Malondialdehyde (μM) | 4.42 | 4.63 | 4.00 | 2.56 | 3.53 | 5.26 | 0.98 | 0.285 |
| Protein carbonyl (nmol/mg) | 1.59 | 1.50 | 0.72 | 1.03 | 1.29 | 0.76 | 0.22 | 0.122 |
| 8-hydroxy-deoxyguanosine (ng/mL) | 0.71 | 1.00b | 0.90b | 1.07b | 1.04b | 0.48a | 0.12 | <0.001 |
1) HT, high thermal environment (average daily minimum and maximum temperatures of 24.8°C±2.2°C, 30.3°C± 2.9°C in gestation building, and 22.1°C±1.8°C, 30.9°C±2.6°C in farrowing building, respectively); MT, moderate thermal environment (average daily minimum and maximum temperatures of 11.9°C±3.0°C, 16.7°C±3.5°C in gestation building, and 20.4°C±2.1°C, 22.3°C±2.0°C in farrowing building, respectively).
2) Oxidative stress data from d 35 of gestation was used as a covariance when analyzing the oxidative stress indicators.
Table 3
| Item | Exp. 1 | SD2) | Exp. 2 | SD2) |
|---|---|---|---|---|
| n3) | 11 | 12 | ||
| Parity | 5.8 | 3.2 | 5.1 | 1.3 |
| Body weight of sows (kg) | ||||
| d 35 of gestation | 246 | 39 | 243 | 30 |
| d 109 of gestation | 281 | 35 | 290 | 27 |
| d 1 of lactation | 275 | 32 | 276 | 28 |
| d 18 of lactation | 273 | 29 | 261 | 31 |
| Body weight changes (kg) | ||||
| Gestation (d 35 to 109) | 35 | 17 | 47 | 14 |
| Lactation (d 1 to 18) | −5 | 13 | −13 | 17 |
| Backfat of sows4) (mm) | ||||
| d 1 of lactation | 17.0 | 2.5 | 15.8 | 3.9 |
| d 18 of lactation | 16.6 | 2.9 | 14.4 | 3.8 |
| Change from d 1 to 185) | −0.4 | 2.7 | −1.4 | 1.6 |
| ADFI of sows (kg) | 4.3 | 1.1 | 4.6 | 1.1 |
| Litter size (pig) | ||||
| d 1, total born | 12.5 | 2.9 | 13.6 | 2.2 |
| d 1, born alive | 9.5 | 2.9 | 11.9 | 1.8 |
| d 1, stillborn | 2.5 | 1.9 | 1.2 | 1.2 |
| d 1, mummy | 0.5 | 0.8 | 0.4 | 0.9 |
| d 18 | 7.4 | 2.1 | 10.4 | 1.2 |
| Change from d 1 to 185) | −2.2 | 1.7 | −1.5 | 1.7 |
| Litter weight (kg) | ||||
| d 16) | 15.0 | 3.7 | 17.9 | 2.9 |
| d 18 | 42.1 | 11.7 | 55.4 | 11.2 |
| Gain from d 1 to 185) | 27.1 | 8.7 | 37.5 | 9.5 |
| Piglet weight (kg) | ||||
| d 17) | 1.66 | 0.42 | 1.52 | 0.20 |
| d 18 | 5.78 | 0.96 | 5.42 | 0.78 |
| ADG from d 1 to 18 (g/d) | 204 | 41 | 200 | 41 |
1) HT, high thermal environment (average daily minimum and maximum temperatures of 24.8°C±2.2°C, 30.3°C±2.9°C in gestation building, and 22.1°C±1.8°C, 30.9°C±2.6°C in farrowing building, respectively); MT, moderate thermal environment (average daily minimum and maximum temperatures of 11.9°C±3.0°C, 16.7°C±3.5°C in gestation building, and 20.4°C±2.1°C, 22.3°C±2.0°C in farrowing building, respectively).
Table 4
| Item | Plasma | SEM | p value | ||
|---|---|---|---|---|---|
|
|
|||||
| d 109 of gestation | d 1 of lactation | d 18 of lactation | |||
| Exp. 1 | |||||
| n2) | 11 | 11 | 11 | - | - |
| IgM (mg/mL) | 3.25 | 2.55 | 2.33 | 0.41 | 0.419 |
| IgG (mg/mL) | 19.38 | 17.98 | 19.67 | 1.69 | 0.898 |
| Exp. 2 | |||||
| n2) | 12 | 12 | 12 | - | - |
| IgM (mg/mL) | 2.96 | 2.36 | 2.53 | 0.28 | 0.621 |
| IgG (mg/mL) | 19.20ab | 16.22a | 20.07b | 1.19 | 0.031 |
1) HT, high thermal environment (average daily minimum and maximum temperatures of 24.8°C±2.2°C, 30.3°C± 2.9°C in gestation building, and 22.1°C±1.8°C, 30.9°C±2.6°C in farrowing building, respectively); MT, moderate thermal environment (average daily minimum and maximum temperatures of 11.9°C±3.0°C, 16.7°C±3.5°C in gestation building, and 20.4°C±2.1°C, 22.3°C±2.0°C in farrowing building, respectively).
Table 5
| The first set of variables1) | The second set of variables2) | Canonical correlation coefficient | p-value | Canonical variable composition |
|---|---|---|---|---|
| Oxidative stress indicators | Sow BW | 0.990 | 0.023 |
V1 = 0.924Md60 + 0.340Pd60 + 0.652Od60 W1 = 0.364X1 − 1.222X2 |
| Oxidative stress indicators | Sow backfat | 0.879 | 0.093 |
V1 = 1.211Md90 + 1.143Pd90 + 0.621Od90 W1 = 0.277X3 − 1.026X4 |
| Oxidative stress indicators | Piglet weight | 0.960 | 0.059 |
V1 = −0.988Md18 − 1.448Pd18 − 0.413Od18 W1 = 0.269X5 + 0.821X6 |
Table 6
| The first set of variables1) | The second set of variables2) | Canonical correlation coefficient | p-value | Canonical variable composition |
|---|---|---|---|---|
| Oxidative stress indicators | Piglet weight | 0.999 | 0.024 |
V1 = 0.482Md3 − 0.215Pd3 + 0.823Od3 W1 = −0.187X5 − 0.986X6 |
| Oxidative stress indicators | Sow litter size | 0.999 | 0.023 |
V1 = 0.181Md90 − 1.029Pd90 + 0.077Od90 W1 = 0.257X7 + 0.889X8 |
REFERENCES
- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





