Effects of zearalenone on the localization and expression of the growth hormone receptor gene in the uteri of post-weaning piglets
Article information
Abstract
Objective
In this study, we investigated the adverse effects of dietary zearalenone (ZEA) (0.5 to 1.5 mg/kg diet) on the localization and expression of the growth hormone receptor (GHR) in the uteri of post-weaning gilts and explored alternative mechanism of the reproductive toxicity of ZEA on piglets.
Methods
A total of forty healthy piglets (Duroc×Landrace×Large White) aged 28 d were selected for study. Piglets were transferred to single cages after 10 days’ adaptation on an obstetric table. The animals were allocated to one of four treatments: a normal basal diet supplemented with 0 (Control), 0.5 (ZEA0.5), 1.0 (ZEA1.0), or 1.5 (ZEA1.5) mg/kg purified ZEA, and fed for 35 d after the 10-d adaptation. Analyzed ZEA concentrations in the diets were 0, 0.52±0.07, 1.04±0.03, and 1.51±0.13 mg/kg, respectively. At the end of the feeding trial, piglets were euthanized after being fasted for 12 h. Two samples of uterine tissue from each pig were rapidly collected, one of which was stored at −80°C for analysis of the relative mRNA and protein expression of GHR, and the second was promptly fixed in Bouin’s solution for immunohistochemical analysis.
Results
The relative weight of the uteri and thickness of the myometrium and endometrium increased linearly (p<0.001) and quadratically (p<0.001) with an increasing level of ZEA. The results of immunohistochemical analysis indicated that GHR immunoreactive substance was mainly localizated in the cytoplasm of uterine smooth muscle, glandular epithelial, luminal epithelial, stromal, and vascular endothelial cells. In contrast, nuclear staining was rarely observed. The immunoreactive integrated optic density of GHR in the myometrium, luminal epithelium, glandular epithelium, and whole uteri of weaning gilts increased linearly (p<0.001) and quadratically (p<0.05) with an increasing level of ZEA. The mRNA and protein expression of GHR in the uteri of weaning gilts increased linearly (p<0.001) and quadratically (p<0.05) with an increasing level of ZEA.
Conclusion
In conclusion, ZEA at a concentration of 0.5 mg/kg was sufficient to significantly thicken the myometrium and endometrium, and at a concentration of 1.0 mg/kg induced a high level of GHR expression to promote growth and development of the uteri. This revealed an alternative molecular mechanism whereby ZEA induces growth and development of the uteri and provides a theoretical basis for the revision of Chinese feed hygiene standards.
INTRODUCTION
The mycotoxin zearalenone (ZEA) is an estrogenic metabolite produced by a variety of species of Fusarium and Gibberella molds [1–4]. ZEA is commonly found in grain crops (e.g., corn, barley, sorghum, rye, and wheat) and feeds originated from animal sources (e.g., meat and dairy products) [5,6]. It has previously been reported that severe contamination of grain, processed grain by-products, and feed with ZEA occurs in numerous areas of the world [7,8]. Pigs, and in particular female pigs, are sensitive to ZEA [9], and the European Committee has limited its concentration to 0.1 mg/kg in piglet diets [10], whereas in China, the maximum limit of ZEA in diets is 0.5 mg/kg [11]. This mycotoxin as well as its main metabolites, including α-zearalenol (α-zol), β-zearalenol (β-zol), α-zearalanol (α-zel), and β-zearalanol (β-zel), have been shown to have estrogenic activity due to their structural similarity to 17β-estradiol [12–14]. Research has shown that 50 to 100 mg/kg ZEA in diets can cause typical clinical symptoms, including swollen vulva and vaginal prolapse, and also reproductive disorders such as ovarian abnormalities, pseudopregnancy, infertility, miscarriage, and false estrus in sows [15]. Diets supplemented with 1.1, 2.0, and 3.2 mg/kg ZEA have been shown to increase the index of reproductive organs in piglets, stimulate swelling of the vulva, and alter the structure of the uteri and ovaries [14].
Growth hormone (GH), a single-chain polypeptide hormone secreted by the pituitary gland of animals, plays an indispensable role in the growth and metabolism of the body by regulating the metabolism of carbohydrates, lipids, and proteins [16,17]. As the main hormone of the growth axis, GH plays an important role in regulating the growth and development of animals [18]. Growth hormone receptor (GHR) is the receptor protein of GH. As a biological macromolecule, GH must combine with GHR on the target cell membrane and transfer information to the cell through a dielectric conductor to fulfil its biological function [19]. Although there have been many reports on the expression of GHR in the uteri during the estrous cycle of rats [20], pigs [21], and sheep [22], there is little information available on the negative effect of low concentrations of ZEA (0.5 to 1.5 mg/kg) on the localization and expression of the GHR in post-weaning piglets. In the present study, we therefore sought to examine whether feeding a ZEA-contaminated (0.5, 1.0, and 1.5 mg/kg) diet to post-weaning piglets would influence of the localization of GHR, and affect GHR mRNA and protein expressions.
MATERIALS AND METHODS
Preparation of zearalenone-contaminated diet
Purified crystalline ZEA (Fermentek, Jerusalem, Israel) was dissolved in acetic ether and then poured onto talcum powder. The material was left overnight to allow acetic ether evaporation and a ZEA premix of 1,000 mg/kg was subsequently prepared. This premix was then diluted to 10 mg/kg ZEA premix with toxin-free corn meal. The experimental diets were formulated with 10 mg/kg ZEA premix instead of corn and talcum powder carrier according to the required level of ZEA and stored in covered containers. The doses of ZEA (0, 0.5, 1.0, and 1.5 mg/kg) used in the present study were based on the results of Jiang et al [14], Chen et al [23], and Dai et al [24]. Experimental diet preparation was completed a week before conducting the trial. The toxin levels in the diet were examined immediately by the Qingdao Entry Exit Inspection and Quarantine Bureau after sampling before and at the end of the feeding experiment. Deoxynivalenol (DON) was analyzed using high-performance liquid chromatography and an enzyme-linked immunosorbent assay, and fluorescent techniques were used to measure ZEA, fumonisins (FUM), and aflatoxin (AFL) levels. The detection limits for AFL, ZEA, DON, and FUM were 1.0 μg/kg, 0.1 mg/kg, 0.1 mg/kg, and 0.25 mg/kg, respectively.
Experimental design, female piglets, and management
The piglets used in all experiments were cared for in accordance with the guidelines for the care and use of laboratory animals prescribed by the Animal Nutrition Research Institute of Shandong Agricultural University and the Ministry of Agriculture of China. A total of 40 healthy post-weaning piglets (Duroc×Landrace×Large White) aged 28 d were selected for this study. After 10 days’ adaptation on an obstetric table, piglets were transferred to single cages (0.48 m2) fitted with a plastic slatted floor and nipple drinker. The animals were then allocated to one of four treatments. Each treatment consisted of 10 replicates of one pig per replicate according to the average body weight 14.01±0.86 kg (mean±standard deviation [SD]). Piglets were fed a basal diet according to NRC [25] (Table 1) supplemented with 0 (Control), 0.5 (ZEA0.5), 1.0 (ZEA1.0), or 1.5 (ZEA1.5) mg/kg purified ZEA for 35 d after the 10-d adaptation. Analyzed ZEA concentrations in the diets were 0, 0.52±0.07, 1.04±0.03, and 1.51±0.13 mg/kg, respectively. In all treatment diets, no other toxins were detected. Representative samples of feed were taken at the beginning and end of the experimental period for nutrient analyses according to the methods described by the AOAC [26]. The animal feeding experiment was carried out in the Animal Nutrition Research Institute of Shandong Agricultural University of China. Before the start of the experiment, the pigs’ cages and the surrounding environment were cleaned and disinfected. During the first week of the experiment, the room temperature was maintained at 30°C, and was thereafter maintained at 26°C to 28°C. The relative humidity was approximately 65%.
Sample collection
At the end of the feeding trial, piglets were euthanized after being fasted for 12 h. Uteri were then rapidly isolated from the surrounding fat and tissue under sterile conditions and weighed to calculate the relative weight (relative weight of uteri [g/kg] = uterine weight/live pig weight). Two samples of uterine tissue from each pig were rapidly collected, one of which was collected in an RNase-free 2-mL frozen tube and placed in liquid nitrogen, and then stored at −80°C for subsequent analysis of the relative mRNA expression of GHR. The second sample was promptly fixed in Bouin’s solution for immunohistochemical analysis. Following fixation, 5-μm sections were cut on a Leica RM 2235 microtome (Leica, Germany), mounted on poly-L-lysine-coated glass slides, and dried overnight at 37°C prior to routine staining for immunohistochemical analysis.
Immunohistochemistry
Sections were dewaxed and rehydrated, and antigen retrieval was performed in sodium citrate buffer (0.01 mol/L, pH 6.0) using a microwave unit for 20 min at full power. The sections were then washed (3×5 min) with phosphate buffer saline (PBS) (0.01 mol/L, pH 7.2). Endogenous peroxidase activity was blocked by incubating sections in 10% hydrogen peroxide (H2O2) for 1.5 h. To block nonspecific binding, sections were incubated for 1 h in 10% normal goat serum (ZSGB-BIO, Beijing, China). Immunohistochemical analysis was conducted according to kit instructions (Histostain-SP Kits for rabbit primary antibody, SPN-9001, ZSGB-BIO, Beijing, China). After washing with PBS, the sections were incubated overnight with polyclonal rabbit antibody GHR (1:80, bs-0654R, BIOSS, Beijing, China) at 4°C. The following day, the sections were washed with PBS and were subsequently incubated with biotinylated secondary antibody (anti-rabbit immunoglobulin G [IgG]; 1:150) for 2 h at 37°C, and then incubated with horseradish peroxidase label (1:150) for 1 h. The sections were subsequently washed with PBS, followed by immersion in diaminobenzidine tetrachloride (DAB kit, TIANGEN PA110, Beijing, China) for 1 to 3 min to detect immunostaining. The sections were counterstained with hematoxylin, followed by color separation with acid alcohol, and then submerged in tap water until the sections turned blue. The sections were then dehydrated, sealed in clear resin, mounted, and observed microscopically for the distribution of positive cells using a bright field of view.
Measurement of the integrated optical density of the growth hormone receptor immunohistochemistry
Histological sections of the uteri were observed using a microscope (Nikon ELIPSE 80i, Tokyo, Japan) at magnifications of ×40, ×100, and ×200. To evaluate the amount of cell staining and quantity of the target antigen of GHR, the images were analyzed using image analysis software (Image Pro-Plus 6.0, Media Cybernetics, Sliver Spring, MD, USA). This yielded values of the total cross-sectional integrated optical density (IOD) [27], which were used to compare the amount of GHR staining in different parts of the uteri of GHR in the different treatments. We examined at least five stained sections, which were randomly selected from the 10 piglets in each group.
Total RNA extraction, cDNA preparation, and quantitative real-time reverse transcription polymerase chain reaction
Total RNA was extracted from piglet uteri using RNAiso Plus (Applied TaKaRa, DaLian, China), following the manufacturer’s directions. The purity and concentration of the RNA was assessed using an Eppendorf Biophotometer (Eppendorf, RS323C, Leipzig, Germany) at an absorbance ratio of 260/280 nm (values in the range 1.8 to 2.0 indicate a pure RNA sample). RNA integrity was verified by agarose gel electrophoresis. Total RNA was reverse transcribed to cDNA using a Reverse Transcription System kit (PrimeScript RT Master Mix, RR036A, Applied TaKaRa, China).
For quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR), the total volume of the PCR reaction mixture was 20 μL, which contained SYBR Premix Ex Taq-TIi RNaseH Plus (TaKara code: RR420A, Lot: AK7502, China). Each sample was analyzed in three replicates. The optimized qRT-PCR protocol included an initial denaturation step at 95°C for 30 s, followed by 43 cycles at 95°C for 5 s, 60°C for 34 s, 95°C for 15 s, and 60°C for 60 s, with a final step at 95°C for 15 s. The qRT-PCR reactions were conducted in an ABI 7500 Real Time PCR System (Applied Biosystems, Foster, CA, USA). The relative amounts of GHR mRNA were expressed and calculated as being equal to 2−ΔΔCT [28]. The primer sequences and product lengths are presented in Table 2.
Western blotting
The total protein of uterine tissue was extracted by the lysate instructions (containing phenylmethanesulfonyl fluoride [PMSF], Beyotime, Shanghai, China) and detected using a BCA protein assay kit (Tiangen Biotech, China). The sample size was 50 μg protein per sample. Samples were separated by electrophoresis on polyacrylamide gels, and were subsequently transferred to nitrocellulose membranes. The membranes were incubated in 5% skimmed milk powder for 2 h, and then washed with Tris buffered saline Tween (TBST) (pH 7.6) (3×10 min), followed by addition of the primary antibody (polyclonal rabbit anti GHR, 1:300 (diluted by primary antibody dilution buffer, Beyotime, China), BIOSS, Beijing; monoclonal anti Actin, 1:1,000, Beyotime, China) and incubated at 4°C overnight. After washing with TBST, the nitrocellulose membranes were then incubated with anti-rabbit IgG antibody (1:3,000 [diluted by secondary antibody dilution buffer, Beyotime, China]; CWBIO) and anti-mouse IgG (1:3,000 [diluted by secondary antibody dilution buffer, Beyotime, China]; CWBIO) for 2.5 h at 37°C. Following washing with TBST, membranes were immersed in a high-sensitivity luminescence reagent (BeyoECL Plus, Beyotime, China), exposed to film using FusionCapt Advance FX7 (Fusion FX, OSTC), and analyzed using Ipp 6.0 (Image Pro-Plus 6.0, Media Cybernetics, USA).
Statistical analysis
Data of relative weight, myometrial and endometrial thickness, IOD, and GHR mRNA and protein expression were subjected to analysis of variance using the general linear model procedure of SAS 9.2. The data were initially analyzed as a completely randomized design with individual piglets as random factors to examine the overall effect of treatments. Orthogonal polynomial contrasts were then used to determine linear and quadratic responses to the ZEA levels of treatments. Significant differences among treatments were further analyzed using Duncan’s multiple range tests. Data were expressed as the mean±SD. All statements of significance are based on a probability of p<0.05.
RESULTS
The relative weight of the uteri and thickness of the myometrium and endometrum
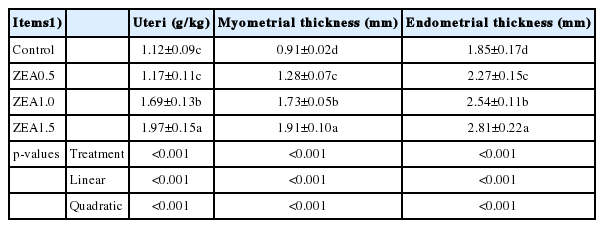
Results of the relative weight of the uteri and thickness of the myometrium and endometrium are shown in Table 3. The relative weight of the uteri and the thickness of the myometrium and endometrium showed significant linear (p<0.001) and quadratic (p<0.001) increases with an increasing level of ZEA. The relative weight of the uteri in the ZEA1.5 treatment was significantly higher than that in the ZEA1.0 treatment (p<0.05), and that in the ZEA1.0 treatment was significantly higher than that in the control and ZEA0.5 treatments (p<0.05). The thickness of the myometrium and endometrium in the ZEA1.5 treatment was significantly higher than that in the ZEA1.0 treatment (p<0.05), whereas that in the ZEA1.0 treatment was significantly higher than that in the ZEA0.5 treatment (p<0.05), and that in the ZEA0.5 treatment was significantly higher than that in the control (p<0.05).
The localization of the growth hormone receptor
The effects of ZEA on GHR localization in the uteri of post-weaning gilts are shown in Figure 1. The results of immunohi-stochemistry indicated that GHR immunoreactive substance was mainly localized in the cytoplasm of smooth muscle cells (M), glandular epithelial cells (G), luminal epithelial cells (LE), stromal cells (S), and vascular endothelial cells (V) in the uteri of piglets. In contrast, nuclear staining was rarely observed. A light yellow immunoreactive substance was observed in the control. The localization pattern of GHR-positive substances in the ZEA-treated pigs was essentially the same as that in the control group (the arrows); however, compared with the control, the positive reaction of GHR was enhanced (A1-B1-C1-D1 and A4-B4-C4-D4), and block localization of yellow and brown immunoreactive substances was observed with an increasing level of ZEA (A2-B2-C2-D2).

Effects of zearalenone (ZEA) on the growth hormone receptor (GHR) localization in the uteri of post-weaning gilts. Control (A), ZEA0.5 (B), ZEA1.0 (C) and ZEA1.5 (D) represent the control diet with an addition of 0, 0.5, 1.0, and 1.5 mg/kg ZEA, and with analyzed ZEA concentrations of 0, 0.52±0.07, 1.04±0.03, and 1.51±0.13 mg/kg, respectively. The 1:40, 1:100, and 1:200 represent the view of the samples in 40, 100, and 200 times, respectively. The dotted line represents the thickness of myometrium, and the line represents the thickness of endometrial. The arrow represents the immunoreactivity of GHR. LE was luminal epithelium, G was uterine gland, M was myometrium, S was stromal cells, V was vessel, and LP was lamina propria.
Immunoreactive integrated optic density of the growth hormone receptor
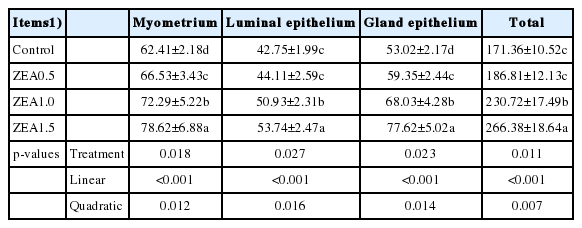
The IOD of GHR in the myometrium, luminal epithelium, glandular epithelium, and whole uteri of weaning gilts showed significant linear (p<0.001) and quadratic (p<0.05) increases with an increasing level of ZEA (Table 4). In general, the IOD of the ZEA1.5 treatment was significantly higher than that of the ZEA1.0 treatment (p<0.05), and that of the ZEA1.0 treatment was significantly higher than that of the ZEA0.5 and control treatments (p<0.05). The IOD values for the myometrium, luminal epithelium, and glandular epithelium in the ZEA1.5 treatment were significantly higher than those of the ZEA1.0 treatment (p<0.05), whereas the IOD values for the myometrium, luminal epithelium, and glandular epithelium in the ZEA1.0 treatment were significantly higher than those of the ZEA0.5 treatment (p<0.05), and the IOD values of the myometrium and glandular epithelium in the ZEA0.5 treatment were significantly higher than those of the control (p<0.05).
Relative mRNA expression of the growth hormone receptor
The results of the analysis of relative mRNA expression of GHR were consistent with those of immunohistochemistry (Figure 2), with the relative mRNA expression of GHR in the uteri of weaning gilts showing significant linear (p<0.001) and quadratic (p<0.001) increases with an increasing level of ZEA. Although the difference between expression levels in the control and the ZEA0.5 treatment was not significant (p>0.05), expression in the ZEA1.5 treatment was significantly higher than that in the ZEA1.0 treatment (p<0.05), and expression in the ZEA1.0 treatment was significantly higher than that in the control and ZEA0.5 treatments (p<0.05).
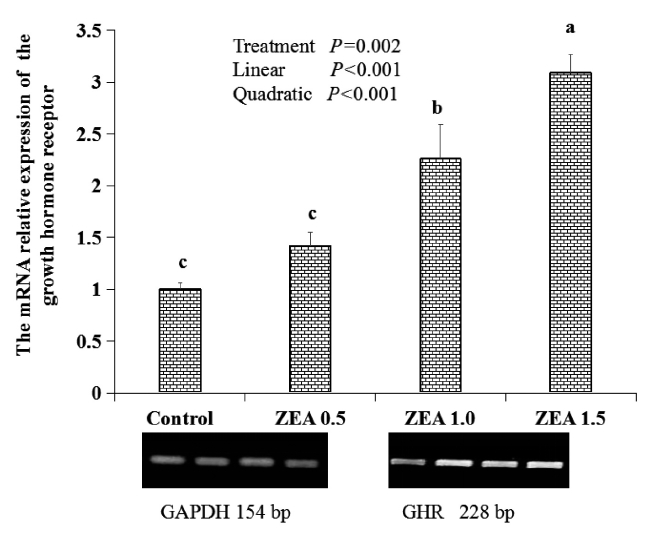
Effects of zearalenone (ZEA) on the relative mRNA expression of the growth hormone receptor (GHR) in the uteri. Control, ZEA0.5, ZEA1.0, and ZEA1.5 represent the control diet with an addition of 0, 0.5, 1.0, and 1.5 mg/kg ZEA, and with analyzed ZEA concentrations of 0, 0.52±0.07, 1.04±0.03, and 1.51±0.13 mg/kg, respectively.
Protein expression of the growth hormone receptor
Western blot analysis was conducted to confirm the specificity of antibodies used. The relative protein expression of GHR in the uteri of weaning gilts increased linearly (p<0.001) and quadratically (p<0.05) with the increasing level of ZEA (Figure 3). Although the difference between control and ZEA0.5 treatment was not significant (p>0.05), protein expression in the ZEA1.5 and ZEA1.0 treatments was significantly higher than that in the ZEA0.5 and control treatments (p<0.05).
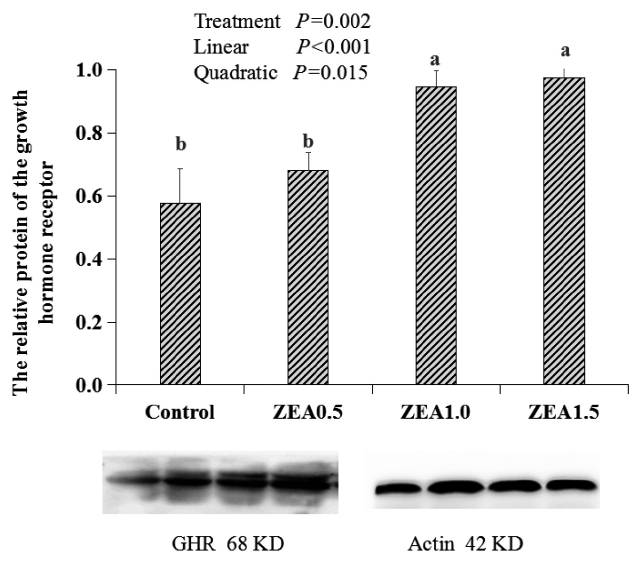
Effects of zearalenone (ZEA) on the relative protein of the growth hormone receptor (GHR) in the uteri. Control, ZEA0.5, ZEA1.0, and ZEA1.5 represent the control diet with an addition of 0, 0.5, 1.0, and 1.5 mg/kg ZEA, and with analyzed ZEA concentrations of 0, 0.52±0.07, 1.04±0.03, and 1.51±0.13 mg/kg, respectively.
DISCUSSION
Most of the previous studies on ZEA toxicity have been performed using diets naturally contaminated with certain levels of ZEA [29], however, naturally contaminated diets may also contain other toxins that could have adverse effect on animals. In the present study, we added high-purity ZEA to the diet, based on our previous research results [14,23,24,30,31], to further explore the influence of low doses of ZEA (0 to 1.5 mg/kg) on GHR localization and GHR mRNA and protein expression in the uteri.
ZEA has estrogenic effects, and mainly affects the reproductive organ of female animals. Fusarium toxins in diets fed to post-weaning gilts (ZEA, 0.90 mg/kg, DON, 1.43 mg/kg, FUM, 5.85 mg/kg) have been shown to induce hyperplasia of the glandular endometrium and myometrium [31]. Addition of ZEA (1.1, 2, and 3.2 mg/kg) in the diet resulted in a dose-dependent proliferation of myometrial smooth muscle cells in weaning-piglets [32]. The results of the present experiment showed that the thickness of the myometrium and endometrium underwent significant linear and quadratic increases in response to an increasing level of ZEA. The thickness of the myometrium and endometrium in the ZEA1.5 and ZEA1.0 treatments was significantly higher than that in the control. In this regard, the results of the present study are consistent with those of previous studies.
On the basis of an analysis of its chemical structure, it has been predicted that ZEA has potential growth-promoting effects [33]. Zearalenol (ZOL), which is formed following the catalytic hydrogenation of ZEA, assimilated into estrogen. It is accordingly used as a growth promoter in ruminant production [34]. The development of the uteri is closely related to GH [35]. Feeding mice a diet containing 1 and 3 mg/kg ZEA has been shown to significantly increase uteri weight [36]. Similarly, in the present study, the relative weight of the uteri in the ZEA1.5 and ZEA1.0 treatments was significantly higher than that in the ZEA0.5 and control treatments. The uteri is the site of GH synthesis and action, and expression of GH and GHR are observed in the uteri of a variety of animals [19,37–39]. Knockout of GHR in mice has been shown to delay the date of sexual maturation and to influence reproductive function [40]. Furthermore, analysis of the relative mRNA expression of GHR, insulin-like growth factor I receptor (IGF-IR), and estrogen receptor-α (ER-α) in the uteri of laying hens has indicated that GH, IGF-I, and estrogen are involved in regulation of the development and function of the uteri [41]. The close relationship between the expression of GHR in the uteri and its growth and development was discovered by Tong [42], who examined the localization and RNA expression of GHR in the uteri of Jining Gray Goats. Immunoreactive substance was mainly localized in the cytoplasm of glandular epithelial cells, smooth muscle cells, vascular endothelial cells, and uterine smooth muscle cells, together with occasional observations of nuclear coloration [42]. The results of our immunohistochemical analysis indicated that GHR immunoreactive substance was mainly localized in the cytoplasm of uterine smooth muscle cells, glandular epithelial cells, luminal epithelial cells, stromal cells, and vascular endothelial cells. In contrast, nuclear staining was rarely observed. A light yellow immunoreactive substance was observed in the control, whereas a positive GHR reaction was enhanced and block distribution of yellow and brown immunoreactive substance was observed with an increasing level of ZEA. The localization pattern of GHR observed in the present study differed from that observed by Tong [42], which can presumably be explained by the toxicity of ZEA and the different species of study animals.
The mechanism underlying the effect of ZEA on the development of reproductive organs in animals is primarily related to its estrogenic properties. ZEA and its metabolites are agonists of ERα (they are observed mainly in the uteri, but not in the ovaries) [43]. The addition of 0.5 to 2 mg/kg ZEA in the diet has been shown to significantly increase the transcription level of ERα in the uteri and vaginal tissue of gilts, whereas it significantly reduced the transcription level of ERβ, which regulates the development of reproductive organs [44]. The mechanism whereby ZEA acts on sow uteri can probably be explained by the fact that α-ZOL has a high affinity for endometrial estrogen receptors, and competes with estrogen for target tissue receptors to promote the synthesis of DNA, RNA, and protein. [45].
In this study, we investigated GHR, which plays a key role in the growth and development of the uteri. The results showed that the immunoreactive IOD and mRNA and protein expressions of GHR in the uteri of piglets increased linearly with an increasing level of ZEA, which suggests that ZEA can promote uterine development to some extent. It is worth noting that the relative weight of the uteri and mRNA expression of GHR in the ZEA1.0 and ZEA1.5 treatments were significantly higher than those in the ZEA0.5 and control treatments, leading us to speculate that the reproductive toxicity of ZEA is mediated via another growth mechanism. The specific molecular mechanism by which GHR promotes uterine development is accordingly in need of further study.
ACKNOWLEDGMENTS
This research was financed in part by National Nature Science Foundation of China (Project No. 31572441), Natural Science Foundation of Shandong Province (Project No. ZR2017MC049), Agriculture Research System in Shandong Province (SDAIT-08-04) and Founds of Shandong “Double Tops”.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.