Effects of dietary energy sources on early postmortem muscle metabolism of finishing pigs
Article information
Abstract
Objective
This study investigated the effects of different dietary energy sources on early postmortem muscle metabolism of finishing pigs.
Methods
Seventy-two barrow (Duroc×Landrace×Yorkshire, DLY) pigs (65.0±2.0 kg) were allotted to three iso-energetic and iso-nitrogenous diets: A (44.1% starch, 5.9% crude fat, and 12.6% neutral detergent fibre [NDF]), B (37.6% starch, 9.5% crude fat, and 15.4% NDF) or C (30.9% starch, 14.3% crude fat, and 17.8% NDF). After the duration of 28-day feeding experiment, 24 pigs (eight per treatment) were slaughtered and the M. longissimus lumborum (LL) samples at 45 min postmortem were collected.
Results
Compared with diet A, diet C resulted in greater adenosine triphosphate and decreased phosphocreatine (PCr) concentrations, greater activity of creatine kinase and reduced percentage bound activities of hexokinase (HK), and pyruvate kinase (PK) in LL muscles (p<0.05). Moreover, diet C decreased the phosphor-AKT level and increased the hydroxy-hypoxia-inducible factor-1α (HIF-1α) level, as well as decreased the bound protein expressions of HK II, PKM2, and lactate dehydrogenase A (p<0.05).
Conclusion
Diet C with the lowest level of starch and the highest levels of fat and NDF could enhance the PCr utilization and attenuate glycolysis early postmortem in LL muscle of finishing pigs.
INTRODUCTION
The postmortem muscle energy metabolism involved in the process of muscle conversion to meat is tightly correlated with the meat quality [1]. After slaughter, the adenosine triphosphate (ATP), which maintains postmortem muscle contraction, is mainly supplied by the breakdown of phosphocreatine (PCr) and the degradation of glycogen to lactate [2]. The PCr is broken down firstly, and when 70% of the PCr content is consumed, the glycolysis is next in turn to meet the need of ATP and is accompanied by the accumulations of lactate and heat [3]. During postmortem muscle glycolysis, the glycolytic flux and lactate accumulation are regulated by glycolytic enzymes. Moreover, these enzymes exist as soluble or bound forms and differ in their kinetic properties [4]. For example, the activity of bound lactate dehydrogenase (LDH) is not inhibited by high concentration of pyruvate [5]. The bound hexokinase (HK) II in muscle associated with the outer mitochondrial membrane has elevated catalytic activity compared to the soluble enzyme [4]. Furthermore, previous studies reported that the distribution of glycolytic enzymes between soluble and bound forms could be modulated by electrical stimulation and some metabolites such as ATP and adenosine diphosphate (ADP) [6].
Hypoxia-inducible factor-1 (HIF-1) plays an important role in the up-regulation of glycolytic enzymes gene expressions in cells subjected to hypoxia [7] or in skeletal muscle subjected to exercise [8]. The HIF-1 is composed of a constitutively expressed HIF-1β subunit and an O2-dependented HIF-1α subunit [9]. Under normoxia conditions, HIF-1α is regulated by prolyl hydroxylases (PHDs), which target HIF-1α for ubiquitination and proteasomal degradation. However, upon hypoxia, this degradation is prevented via the inactivation of PHDs [10], thereby activating HIF-1 target genes. Besides promoting the mRNA expressions of glycolytic enzymes, the stabilization of HIF-1α can also lead to an increase of protein-protein interactions between HK II and the voltage-dependent anion channel located on the outer mitochondrial membrane [11], thereby improving the association of HK II and mitochondrions [12]. In addition, previous study reported that the activation of HIF-1α could lead to the reorganization of the actin cytoskeleton [13], which might provide an increased surface for binding of glycolytic enzymes [14]. In addition to the hypoxia stimuli, phosphatidylinositol 3-kinase (PI3K)/serine-threonine kinase AKT signaling pathway has also been shown to involve in the stabilization of HIF-1α through inhibiting the PHD2 [15]. In mammalians, insulin can activate the PI3K pathway [11], whilst, plasma insulin level can be regulated by dietary carbohydrate content. Consequently, the present study assumed that different levels of dietary starch and fat might influence the PI3K/AKT-HIF-1α pathway.
Results in our previous study showed that, diets with the lowest levels of starch and the highest levels of fat and neutral detergent fibre (NDF) decreased the average daily gain, average daily feed intake and the muscle lactate concentration without affecting the muscle glycogen concentration at 45 min postmortem, meanwhile improved the meat quality in comparison to diets with the highest levels of starch and the lowest levels of fat and NDF in finishing pigs [16]. It is well established that, the postmortem energy metabolism or glycolysis (lactate) in muscle is highly relevant to the ultimate meat quality, and the muscle glycolytic flux early postmortem is mainly related to the phosphagen system and glycolytic enzymes activities [17]. However, there is limited literature about the effects of dietary energy source on the phosphagen system and the HIF-1α induced distribution of glycolytic enzymes. Therefore, the main aim of this study was to investigate the effects of different compositions of dietary energy sources (starch, crude fat, and NDF) on the phosphagen system and HIF-1α-mediated redistribution of soluble and bound glycolytic enzymes in LL muscle of finishing pigs.
MATERIALS AND METHODS
Animal treatments and experimental diets
The experimental design and procedures were approved by the Animal Care and Use Committee of Nanjing Agricultural University. Animal design, growth performance, carcass data and meat quality of experimental pigs have been described in a joint experiment [16]. Briefly, seventy-two barrow (Duroc×Landrace× Yorkshire, DLY) pigs (average body weight = 65.0±2.0 kg) were uniformly allotted into three isoenergetic and iso-nitrogenous experimental diet treatments, and each treatment consisted of three replicates (pens) of eight pigs each. All diets were formulated to meet the nutrient requirements of National Research Council [18] for finishing pigs. The traditional diet (diet A) of finishing pigs was used as the control diet, the starch content of which was 44.1%. The starch contents of the diets B and C were 37.6% and 30.9%, respectively. The starch content was formulated by decreasing approximately 15% or 30%, on the basis of diet A. In the case of isoenergetic diets, the contents of dietary fat and NDF varied with the change of dietary starch content. Diet A contained 44.1% starch, 5.9% crude fat, and 12.6% NDF; diet B contained 37.6% starch, 9.5% crude fat, and 15.4% NDF; diet C contained 30.9% starch, 14.3% crude fat, and 17.8% NDF. The nutrient composition and the nutrient levels of the experimental diets are shown in Table 1. Diets were fed in mash form and pigs were allowed ad libitum access to feed and water.
Sample collection
After the duration of 28-day feeding experiment, a total of 24 pigs (eight per treatment, two or three per pen near to the average body weight of the pen) were selected after 12 h fasting and transported to a slaughterhouse. Pigs were electrically stunned, exsanguinated, scalded, depilated, labelled, eviscerated, and divided into halves. Hot carcasses were then sent to the chilling room (4°C). In the chilling room, at 45 min postmortem, samples of M. longissimus lumborum (LL) taken at the last rib of the left carcass were immediately frozen in liquid nitrogen for the further analysis of this study.
ATP/ADP/AMP determinations
The contents of adenosine phosphates (ATP, ADP, and adenosine monophosphate [AMP]) in LL muscle were evaluated by high-performance liquid chromatography (HPLC) according to the method of Wang et al [19] as follows. A frozen muscle sample (0.5 g) was ice bath homogenized in 2.5 mL of 7% ice-cold perchloric acid at 13,500 rpm for 30 s and then centrifuged (15,000 g, 4°C, 10 min). The supernatant was neutralized with 0.85 M KOH and centrifuged again (15,000 g, 4°C, 10 min) to remove KClO4. The neutralized supernatant was filtered through a 0.45 μm filter before injection into the Waters-2695 Alliance HPLC system (Waters, Milford, MA, USA). The column was Kromasil 5 μm C18, 250×4.6 mm (Feinano, Tianjin, China). The chromatographic conditions were set as follows: mobile phase A was HPLC grade methanol, mobile phase B was phosphate buffer (2.5 mM tetra-butylammonium hydrogen sulfate, 0.04 M potassium dihydrogen orthophosphate, and 0.06 M dipotassium hydrogen orthophosphate, pH 7.0), which was filtered through a membrane of 0.45 μm, and mobile A/mobile B was 13.5%/86.5%. The column temperature was set at 30°C, and the injection volume was 10 μL and UV detection at 254 nm. The characteristic running time was 15 min and flow-rate was kept at 1.0 mL/min, sample measurement was set to auto-sequence injection. Peaks were identified and quantified by the standard curves. Standard curves establishments were according to the method previously described by Zhang et al [20].
Creatine and phosphocreatine determinations
The contents of creatine (Cr) and PCr in LL muscle were evaluated by HPLC according to the method of Liu et al [21] as follows. A frozen muscle sample (0.3 g) was ice bath homogenized in 2 mL of 5% ice-cold perchloric acid at 13,500 rpm for 30 s and extracted for 15 min. After the homogenate was centrifuged (10,000 g, 4°C, 10 min), the supernatant was neutralized with 0.8 M K2CO3 and centrifuged again (10,000 g, 4°C, 15 min). The neutralized supernatant was then filtered through a 0.45 μm filter before injection into the Waters-2695 Alliance HPLC system (Waters, USA). The column was Kromasil 5 μm C18, 250×4.6 mm (Feinano, China). The chromatographic conditions were set as following: mobile phase A was HPLC methyl cyanides, mobile phase B was phosphate buffer (1.15 mM tetra-butylammonium hydrogen sulfate, 29.4 mM potassium dihydrogen orthophosphate, pH 5.1), which was filtered through a membrane of 0.45 μm, and mobile A/mobile B was 2%/98%. The column temperature was set at 25°C, and the injection volume was 20 μL and UV detection was at 210 nm. The characteristic running time was 20 min and flow-rate was kept at 1.0 mL/min, sample measurement was set to auto-sequence injection. Peaks were identified and quantified by the standard curves. Standard curves establishments were according to the method previously described by Zhang et al [20].
Enzyme activities determinations
To determine the activities of soluble and bound glycolytic enzymes (HK, pyruvate kinase [PK], LDH), the preparation of tissue extracts were according to the procedures of Clarke et al [22]. Briefly, a frozen muscle sample (1 g) was ice bath homogenized in 3 mL of ice-cold 0.25 M sucrose which contained 0.001 mM dithiothreitol at 13,500 rpm for 30 s. And then 1 mL of the homogenate was centrifuged (32,000 g, 4°C, 5 min), 0.5 mL of the resulting supernatant was collected and diluted with 2 mL of a stabilization buffer (0.1 M potassium phosphate, 0.001 M ethylenediaminetetraacetic acid, 0.002 M dithiothreitol, 0.1 mM fructose 1, 6-bisphosphate and 0.1 mM ATP, pH7.5) for soluble enzymes activities analyses. The pellet was extracted with 2×1 mL of stabilization buffer, then the extracts were collected for analyses of bound enzymes activities. Finally, a second 1 mL of the whole homogenate was diluted with 4 mL of stabilization buffer for total enzyme activity (creatine kinase [CK], HK, PK, LDH) analysis. This was to verify the recovery of activities in the soluble and bound fractions.
The protein concentrations in the homogenate, supernatant and the extract of pellet were determined by total protein quantitative assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions of the manufacturer. The activities of HK, PK, and LDH in the homogenate, supernatant and the extract of pellet were determined spectrophotometrically with commercial HK, PK, and LDH kits (Nanjing Jiancheng Bioengineering Institute, China). The CK activity only in the homogenate was determined spectrophotometrically with commercial CK kit (Nanjing Jiancheng Bioengineering Institute, China). All the enzymes activities were normalized by their protein concentration respectively. The percentage binding was calculated as bound/(soluble+bound). The recovery of activities in the soluble and bound fractions compared with total enzyme activity ranged from 85% to 120%.
Protein extraction
Total proteins from frozen LL muscles were extracted using a Total Protein Extract Kit (Nanjing Jiancheng Bioengineering Institute, China). Sarcoplasmic and myofibrillar proteins were extracted as previously described by Lametsch et al [23]. Briefly, a frozen muscle sample (1 g) was ice bath homogenized in 6 mL of ice-cold homogenizing buffer containing 100 mM Tris, pH 8.3, Roche Complete and PhosStop protease inhibitor cocktail tablets (1 and 2 tablets per 50 mL, respectively; Roche, Mannheim, Germany) for 2×30 s at 9,500 rpm and 2×30 s at 13,500 rpm with a 30 s cooling between bursts. Then the homogenates were centrifuged at 4°C for 20 min at 15,000×g, the supernatant was collected for sarcoplasmic protein analysis. The pellet (mainly containing the myofibrillar proteins) was dissolved in 25 mL of 5% sodium dodecyl sulfate (SDS) solution (60°C) and re-homogenate at 9,500 rpm for 30 s and heated at 80°C for 20 min, and then stored at 4°C. The protein concentration was determined by bicinchoninic acid protein assay kit (Nanjing Jiancheng Bioengineering Institute, China), and then the homogenates were adjusted to the same protein concentration, finally, each homogenate was mixed with an equal amount of 2×standard sample loading buffer and heated in boiling water for 5 min and cooled on ice for subsequent Western blot analysis.
Western blot analysis
The protein samples with equal amounts were resolved by electrophoresis (Bio-Rad, Richmond, CA, USA) on 10% (AKT, PKM2, LDHA) or 7.5% (HIF-1α, HKII) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) before being transferred electrophoretically to a polyvinylidene difluoride membrane (Millipore, MA, USA). Membranes were blocked using 5% nonfat milk in tris buffered saline (TBS) containing 200 mM Tris, 137 mM NaCl, 5 mM KCl and 0.1% Tween-20 (TBS-T) for 1 h at room temperature. Membranes were then incubated with primary antibodies overnight at 4°C. After washed 5×6 min for unphosphorylated antibodies or 3×6 min for phosphorylated antibodies in TBS-T at room temperature, membranes were then incubated with secondary antibody conjugated with horseradish peroxidase (1:5,000 in TBS-T) for 2 h at room temperature. Membranes were again washed 5×6 min in TBS-T at room temperature and then visualized using ECL Western blotting reagents (Amersham Bioscience, Piscataway, NJ, USA) and exposed to film (MR; Kodak, Rochester, NY, USA). The primary antibodies for total AKT (1:1,000), phosphor-AKT (Ser473, 1:2,000), total HIF-1α (1:1,000), hydroxy-HIF-1α (Pro564, 1:1,000), HK II (1: 1,000), PKM2 (1:1,000), LDHA (1:1,000) and β-actin (1:1,000) were from Cell Signaling Technology. The densitometric intensities were measured and analyzed using the Quantity-One system (Bio-Rad Laboratories, Hercules, CA, USA). The phosphorylation levels of AKT at Ser473 were expressed as the ratio of P-AKT to T-AKT; the hydroxylation levels of HIF-1α at Pro564 were expressed as the ratio of H-HIF-1α to T-HIF-1α; the protein expression of glycolytic enzyme gene was normalized to the amount of protein β-actin.
Statistical analyses
Data analysis was performed by analysis of variance with the SAS statistical software (version 9.2 for Windows, Institute Inc., Cary, NC, USA). The significant difference among treatments was compared by Tukey test. The results were presented by the mean± standard error. All statements of significance were considered on a p-value less than 0.05. The contents of adenosine nucleotides, creatine and PCr, the activity of CK and the percentage bound activities of glycolytic enzymes were analyzed with pen as the experimental unit (n = 3 [The means of two or three pigs per pen were used to represent the pens]). The protein expressions were analyzed with pig as the experimental unit (n = 3).
RESULTS
ATP/ADP/AMP, Cr, and phosphocreatine concentration
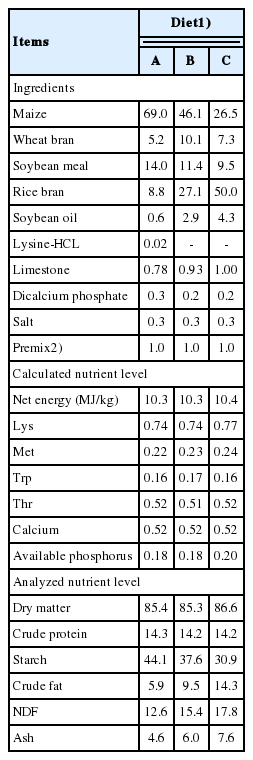
As shown in Figure 1a, pigs fed diets B and C had greater concentration of ATP compared with those fed diet A (p<0.05), whereas no difference in contents of ADP and AMP among three treatments was observed (p>0.05). Conversed to the elevation of ATP, the PCr content was decreased in pigs fed diet C compared with those fed diet A (p<0.05; Figure 1b).
Effects of dietary energy sources on the contents of adenosine nucleotides, creatine and phosphocreatine of M. longissimus lumborum in finishing pigs. (■): diet A, diet contained 44.1% starch, 5.9% crude fat and 12.6% NDF; (□): diet B, diet contained 37.6% starch, 9.5% crude fat and 15.4% NDF; (▨): diet C, diet contained 30.9% starch, 14.3% crude fat and 17.8% NDF. Data are shown as the mean±standard error [n = 3 (The means of two or three pigs per pen were used to represent the pens)]. a,b Mean values in the same target of different treatments were significantly different (p<0.05). NDF, neutral detergent fibre; ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate; Cr, creatine; PCr, phosphocreatine.
Creatine kinase activity and percentage bound glycolytic enzyme activities
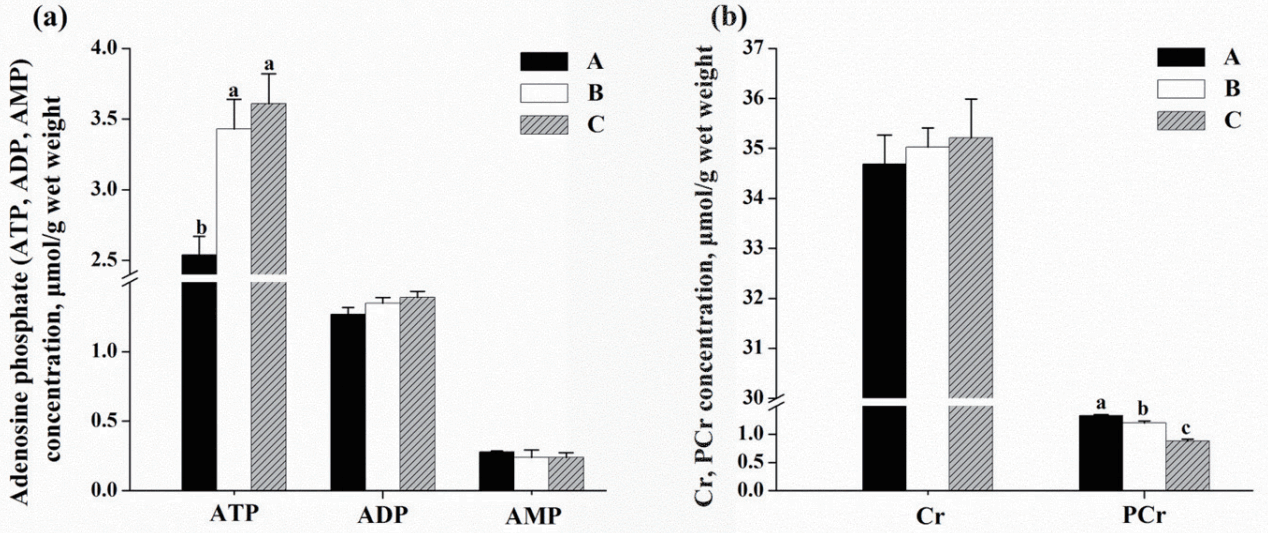
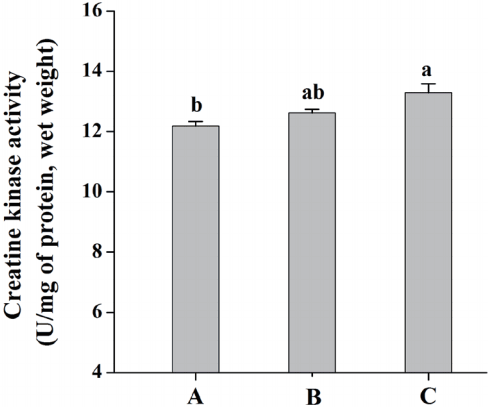
Compared with diet A, pigs fed diet C showed greater activity of CK in LL muscle (p<0.05; Figure 2). And Figure 3 shows the distribution of glycolytic enzymes between soluble and bound form in LL muscle. Pigs fed diet C had a decrease in the percentage bound activities of HK and PK compared with those fed diet A (p<0.05), but diet C did not significantly affect the percentage bound activity of LDH in LL muscle (p>0.05).
Effects of dietary energy sources on creatine kinase activity of M. longissimus lumborum in finishing pigs. Diet A contained 44.1% starch, 5.9% crude fat and 12.6% NDF; diet B contained 37.6% starch, 9.5% crude fat and 15.4% NDF; diet C contained 30.9% starch, 14.3% crude fat, and 17.8% NDF. Data are shown as the mean±standard error [n = 3 (The means of two or three pigs per pen were used to represent the pens)]. a,b Mean values in the same target of different treatments were significantly different (p<0.05). NDF, neutral detergent fibre.
Effects of dietary energy sources on of binding activities of glycolytic enzyemes of M. longissimus lumborum in finishing pigs. (■): diet A, diet contained 44.1% starch, 5.9% crude fat and 12.6% NDF; (□): diet B, diet contained 37.6% starch, 9.5% crude fat, and 15.4% NDF; (▨): diet C, diet contained 30.9% starch, 14.3% crude fat, and 17.8% NDF. Data are shown as the mean±standard error [n = 3 (The means of two or three pigs per pen were used to represent the pens)]. a,b Mean values in the same target of different treatments were significantly different (p<0.05). NDF, neutral detergent fibre; HK, hexokinase; PK, pyruvate kinase; LDH, lactate dehydrogenase. Percentage binding = bound/(bound+soluble).
PI3K/AKT-HIF-1α signaling pathway
To determine whether the redistribution of glycolytic enzymes between soluble and bound forms were associated with the PI3K/AKT-HIF-1α signaling pathway, the phosphorylation level of AKT and hydroxylation level of HIF-1α were examined. Compared with diet A, pigs fed diet C decreased the level of phospho-AKT and increased the level of hydroxylated of HIF-1α, as revealed by western blot and trace quantity analysis of the ratio of phospho-AKT to total AKT (p<0.05; Figure 4a) and the ratio of hydroxyl-HIF-1α to total HIF-1α (p<0.05; Figure 4b).
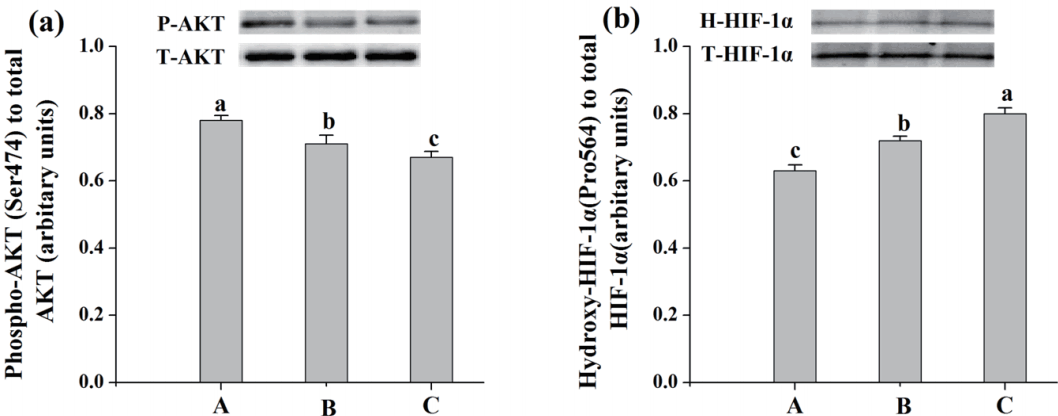
Effects of dietary energy sources on protein expressions about PI3K/AKT-HIF-1α signaling pathway of M. longissimus lumborum in finishing pigs. Diet A contained 44.1% starch, 5.9% crude fat, and 12.6% NDF; diet B contained 37.6% starch, 9.5% crude fat and 15.4% NDF; diet C contained 30.9% starch, 14.3% crude fat, and 17.8% NDF. Bottom panel: bar graphs (a) showed the quantification of P-AKT normalized to T-AKT, and bar graphs (b) showed the quantification of H-HIF-1α normalized to T-HIF-1α. Data were shown as the mean±standard error (n = 3). a,b Mean values in the same protein of different treatments were significantly different (p<0.05). NDF, neutral detergent fibre; AKT, serine/threonine kinase or protein kinase B; HIF-1α, hypoxia inducible factor-1α.
Protein expressions of soluble and bound glycolytic enzymes
To further verify the redistribution of glycolytic enzymes, the protein expressions of HK II, PKM2, and LDHA in sarcoplasmic protein (soluble forms) and myofibrillar proteins (bound forms) were detected. The amounts of bound proteins of HK II, PKM2, and LDHA (Figure 5b, 5d, 5f) were decreased in diet C treatment compared with those in diet A treatment (p<0.05). However, diet treatments did not significantly affect the amounts of soluble proteins of HK II, PKM2, and LDHA (p>0.05; Figure 5a, 5c, 5e).
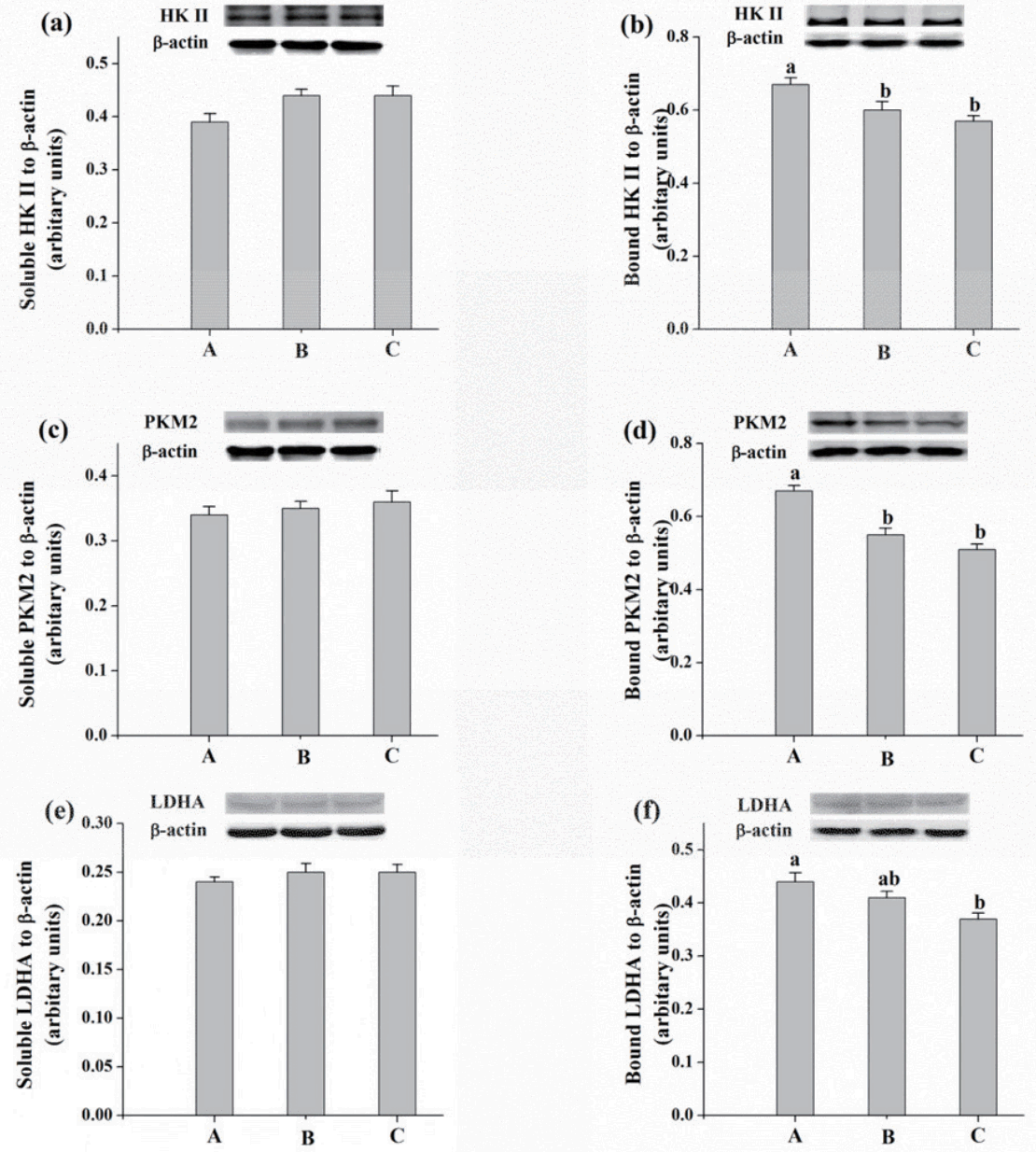
Effects of different energy sources on protein expressions of soluble (a) and bound (b) hexokkinase II (HK II), soluble (c) and bound (d) pyruvate kinase M2 isoform (PKM2), soluble (f) and bound (e) lactate dehydrogenase A isozyme (LDHA) of M. longissimus lumborum in finishing pigs. Diet A contained 44.1% starch, 5.9% crude fat, and 12.6% NDF; diet B contained 37.6% starch, 9.5% crude fat, and 15.4% NDF; diet C contained 30.9% starch, 14.3% crude fat, and 17.8% NDF. Bottom panel: bar graphs show the quantification of target protein normalized to protein β-actin. Data are shown as the mean±standard error (n = 3). a,b Mean values in the same protein of different treatments were significantly different (p<0.05). NDF, neutral detergent fibre.
DISCUSSION
Generally, the postmortem energy metabolism or glycolysis in muscle is highly relevant to the ultimate meat quality, and the muscle glycolytic flux early postmortem is mainly related to the phosphagen system and glycolytic enzymes activities [17]. After slaughter, PCr is initially degraded to provide ATP, and once 70% of the PCr has been consumed, muscle glycolysis is turned on [3]. Therefore, the increase of ATP generated from PCr is advantageous to delay glycolysis early postmortem. Interestingly, the skeletal muscle is unique in its ability to adjust the substrates of energy production in response to the external nutritional status [24]. A previous study has concluded that the increase of fatty acid availability may decrease the glycogenolysis in skeletal muscle [25]. However, carbohydrates can provide more energy per unit of oxygen consumed and release energy more quickly than fat [26].
Anaerobic metabolism (glycolysis) produces two ATP molecules whereas aerobic metabolism produces 38 ATP molecules per glucose molecule [27]. In the present study, pigs fed low starch, high fat, and fibre diet had improved efficiency of ATP production to support muscle growth. This might promote aerobic metabolism in muscle cells, which led to ATP generation. Consistent with our previous study, a diet with low starch promoted the mRNA expressions of oxidative muscle fibre type [16]. Meanwhile, the initial physiological and energy reserves in muscle fibers at slaughter may affect the postmortem muscle metabolism, and adversely influence meat quality [28]. In this study, the result showed an increased content of ATP concurrent with a decreased content of PCr in muscle, which indicated that there was an improved degradation of PCr catalyzed by CK, and therefore a delay of muscle glycolysis. Similarly, Ribeiro et al [29] reported that horses fed with low-starch, high-fat diet had lower post-exercise serum CK activity. As serum CK is released from muscle cells [30], we speculated that a low-starch, high-fat diet might result in higher muscle CK activity, which was consistent with the data in the present study. These results suggested that pigs fed with low starch, high fat and fibre diet might have enhanced utilization of PCr in muscle at postmortem which would then delay muscle glycolysis.
The HIF-1α plays a major role in the shift from muscle oxidative metabolism to glycolysis [7,8]. Fortunately, the stabilization of HIF-1α could be modulated by nonhypoxic stimuli, including insulin-PI3K/AKT pathway [15]. Activation of this pathway starts when the insulin binds to the insulin receptor (InR), and then interacts with InR substrate (IRS) adapter proteins, resulting in the activation of PI3K. The PI3K catalyzes the phosphorylation of phosphatidylinositol-4,5-bisphosphate to phosphatidylinositol-3,4,5-tri-sphosphate (PIP3). The PIP3 promotes recruitment and phosphorylation of AKT/PKB [9], which in turn activates downstream substrates such as HIF-1α. Nutrients including carbohydrate, protein and fat play a role in the in vivo metabolism via their metabolites such as glucose, amino acid and free fatty acid (FFA). One previous study reported that horses fed high-starch, low-fat diet had a higher daily insulin concentration and lower FFA concentration [29]. Additionally, in humans, high content of plasma FFA resulted in inhibition of insulin pathway [31]. We also found in our previous study that a low starch, high fat and fibre diet decreased the plasma insulin content of finishing pigs [32]. Taken together, these above studies could further confirm our results that decreased phosphorylation level of AKT in pigs might be associated with lower plasma insulin concentration in low starch, high fat and fibre diets. Similarly, consistent with the present study, Borges et al [33] also reported that high dietary lipid level resulted in reduction of muscle AKT of fish. Meanwhile, in the present study, an elevated level of hydroxylated of HIF-1α in pigs induced by diet C was detected. It has been known that hydroxyl-HIF-1α was inactive [10], which was in accordance with the upstream down-regulation of phosphor-AKT, as that PI3K/AKT signaling pathway could promote the stabilization of HIF-1α through inhibiting PHD2 [15].
HIF-1α stimulates glycolysis through induction of glucose transporters and glycolytic enzymes (HK II, PKM2, and LDHA) [34]. Glycolytic enzymes, which regulate the glycolytic flux and lactate accumulation, could influence the rate and extent of pH decline in muscle. The decline in muscle pH postmortem has commonly been attributed to the increase in lactic acid, which is strongly linked to the pale, soft and exudative (PSE) meat and a negative impact on meat quality development [17]. The key enzymes of anaerobic glycolysis are HK, phosphofructokinase, and PK. In addition, LDH catalyzes the conversion between pyruvate and lactate involved in postmortem muscle glycolysis, and also has a main function for the muscle anaerobic metabolism.
Glycolytic enzymes exist in muscle as soluble and bound forms which differ in their kinetic properties, the enhanced binding of glycolytic enzymes serve to accelerate glycolytic flux [4]. A fraction of HK II in muscle is associated with the outer mitochondrial membrane and this bound enzyme exhibits several fold elevated catalytic activity compared with the soluble free form [4]. The activity of bound LDH is not inhibited by high concentration of pyruvate [5]. In skeletal muscle, HK II is the predominant HK isoform present, PKM2 is notably associated with the glycogen concentration at early postmortem and LDHA is a particularly abundant type of LDH, therefore we selected the proteins of HK II, PKM2, and LDHA as research objects in the present study. Moreover, in addition to promoting the expression of glycolytic enzymes genes, the HIF-1α could modulate the distribution of glycolytic enzymes via enhancing protein-protein interactions [11] or the reorganization of actin cytoskeleton [13]. In this study, the reduction of anaerobic metabolism (glycolysis) induced by low-starch, high-fat diet, was illustrated by the result of decreased activities of bound enzymes of HK and PK in muscles of pigs fed this diet (Figure 2). To our knowledge, the researches regarding the effects of dietary nutrition on HIF-1α induced the redistribution of glycolytic enzymes are scarce, only a few studies referred to insulin induced distribution of glycolytic enzymes [35]. Nevertheless, Storey [36] summarized that instead of modifying the gene expressions of glycolytic enzymes, the anoxia-induced posttranslational modification could alter subcellular distribution of glycolytic enzymes and then affect their activity state. In addition, the present study showed that the protein expressions of bound form of HK II, PKM2, and LDHA were downregulated in muscle with low activated HIF-1α level of pigs fed low starch, high fat, and fiber diet, which was consistent with the changes of glycolytic enzymes activities. Taken together, we could speculate that the subcellular distribution of glycolytic enzymes may be associated with the HIF-1α to some extent.
CONCLUSION
In summary, the present study demonstrated that diets with the lowest levels of starch and the highest levels of fat and NDF could promote PCr degradation at early postmortem metabolism, decreased muscle glycolytic flux via a reduction of the proportion of bound form of glycolytic enzymes. This reduction of bound enzymes forms may be partially associated with the inhibition of PI3K/AKT-HIF-1α pathway in LL muscle.
ACKNOWLEDGMENTS
This research was funded by National Key Basic Research Program of China (2013CB127306) and the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period of China (2012BAD28B03).
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.