 |
 |
| Anim Biosci > Volume 30(9); 2017 > Article |
|
Abstract
Objective
The present study aimed to investigate whether the long-lasting, recurrent restricting of sows leads to the physiological and psychological reaction of discomfort.
Methods
Sows (Large White) that had experienced restricting for about 0.5 or 3 years and age-matched sows kept in a group housing system (loose sows) were compared. Pupillary light reflex parameters were measured at the weaning stage. Immediately after slaughter, blood samples were taken to measure serum cortisol levels, and the brain was dissected, gene expression in the hippocampus, frontal cortex and hypothalamus was analyzed.
Results
The serum cortisol levels were higher in the confined sows than in the loose sows. The full maturity, but not the young adolescent, confined sows had longer latency time in the onset of pupil constriction than their loose counterparts. Real-time polymerase chain reaction analyses revealed an increased expression of interleukin 6 mRNA in the hippocampus and decreased expression of brain derived neurotrophic factor mRNA in hippocampus and hypothalamus and to a lesser extent in the frontal cortex of the full maturity confined sows, compared with the full maturity loose sows.
In the modern pig production system, confinement such as gestation stalls are a chronic stressor to sows, because space restriction limits expression of innate behaviors of pregnant sows and does not meet their specific needs [1]. Previous studies have shown that long-lasting confinement of sows not only leads to abnormal behavioral responses and physiological reactions [2], but also negatively influences the psychology of sows, causing frustration or depression [3] and seriously affecting the welfare of pigs. From an animal welfare perspective, inappropriate treatment leads first to psychological discomfort, and then to physical discomfort (such as elevated cortisol concentrations) [3]. Mental discomfort can also lead to a decline in immunity; therefore, long-term chronic stress in livestock makes them susceptible to diseases due to the results of reduced immunity [4]. Therefore, if the sow welfare situation is positive, they will be fully satisfied; if their individual physiological and immune status is at a normal level, the sows will be psychologically happy.
Pupillary light reflex (PLR), the contraction response of the pupil to light stimulation [5], is mainly controlled by the sympathetic and parasympathetic nerves of the autonomic nervous system; hence, pupil changes are not influenced by the external environment or psychological stress [6]. Clinical observation found that PLR parameters were different between patients with mental depression and healthy people [7]. In general, the size and responsiveness of the human pupil were governed by the antagonistic action of the sphincter and the dilator muscles of the iris that were controlled by the parasympathetic and the sympathetic nervous system, respectively [8]. The latency and relative contraction amplitude mainly reflected the parasympathetic function [9]. Conventional pupillary reflex studies were focused on the clinical studies of human disease [10], but few reported on animals. In our previous study, we first used the PLR method to evaluate sowsŌĆÖ psychological status and found that confined sows had longer pupillary light reflex time (PLRT) than those of grouped sows [5]. Although the preliminary work had not yet confirmed that PLRT delay was the result of sowsŌĆÖ psychological depression, a large number of human studies had shown that the latency time of the pupil constriction onset of depressive patients was significantly longer than that of normal subjects [11]. Therefore, the measurement of pupil latency time and dynamic parameters during the dilatation and contraction phase of the PLR could effectively determine the sympathetic and parasympathetic nerve function of the central nervous system [12].
The aim of the present study was to investigate whether long-lasting, recurrent confined stress in sows leads to physiological and psychological discomfort reaction. To this end, we researched sows that had experienced restraint for years at a commercial hog farm, together with age-matched animals from another commercial hog farm where sows were kept in an indoor group housing system. The groups of sows had been restrained or loose during one and five of the same reproductive cycles, respectively. We evaluated the persistent effects of long-lasting stress in these sows by measuring the serum cortisol concentrations and the latency time of the pupil constriction, and by assessing interleukin 6 (IL-6) and brain derived neurotrophic factor (BDNF) expression in the hippocampus, frontal cortex and hypothalamus, regions that are considered to be involved in stress regulation, emotional memory, and mood disorders [13]. The comprehensive results will help to evaluate the difference in sowsŌĆÖ physiological and psychological responses between loose and confined system and provide an important scientific basis for improving the welfare of sow production.
The care and use of all sows in this experiment were approved by the Animal Care and Use Committee of the Institute of Animal Sciences, Institutional Animal Care and Ethics. The sows were purchased from two farms, all sows had been bred and delivered by Xinmin pigs (Xinmin Pig Breeding Farm, Harbin, China), i.e. they were from the same genetic line (Large White), bred and reared under the same highly standardized conditions. The study used 80 sows comprising of 40 primiparous sows and 40 high parity sows which were selected at random and average housed in two completely different conditions farms. Sows were grouped by parity, and the following four groups were compared: Young adolescent loose sows and young adolescent confined sows, approximately 0 parity; full maturity loose sows and full maturity confined sows, approximately 5 parity.
Before entering the experimental stage, there was sufficient space at least 2.2 m2 floor space for the young adolescent sows before breeding (10 pigs in a pen at 2.5 m2/pig, pen size was 350 cm├Ś 715 cm). The young adolescent sows were transferred into loose crates or confined crates at approximately 200 days of age with a body weight of 90 to 120 kg. After weaning, the sows immediately returned to the original pens that they inhabited during the gestation period and were prepared to be bred again after 1 to 2 days. In general, full maturity sows would be estrous on 9th day after weaning and start a new round of gestation. The young adolescent sows and full maturity sows from the same group experienced the same stage. In loose experiment, the farrowing pen size was 2,600 cm (L)├Ś1,700 cm (W)├Ś1,500 cm (H), in confined experiment, the farrowing pen size was 210 cm (L)├Ś65 cm (W)├Ś90 cm (H).
At one farm, 20 young adolescent sows and 20 full maturity sows were group-housed during pregnancies, until approximately seven days before farrowing, when they were transferred to farrowing pens, in which straw was provided in the bedding area. The group pens were provided with solid barriers that permitted sows to avoid confrontations and to escape from fights, a measure that is expected to reduce stress during mixing and housing. However, deviating from this schedule, after weaning of the last litter and before transportation to the slaughterhouse, the loose sows were group-housed.
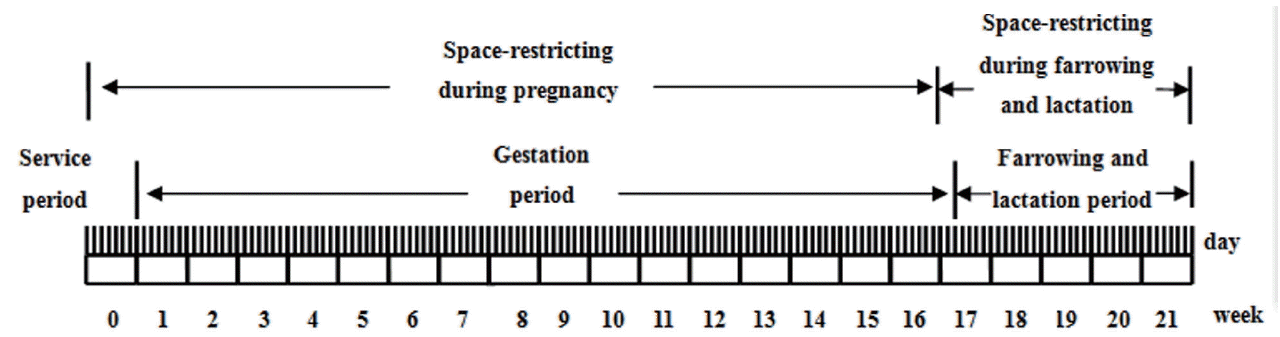
At the other farm, 20 young adolescent sows and 20 full ma turity sows were traditionally stall-housed during pregnancy in pens measuring (210 cm├Ś65 cm├Ś90 cm). The confined sows stayed in the farrowing crate in the period between weaning of the last litter and transportation to the slaughterhouse. The reproductive cycle of confined sows is schematically shown in Figure 1.
The sows were fed twice daily (at 07:30 and 16:00) during the pregnancy and lactation. The sows were fed 3 kg/d during all pregnancy stage, and the quantity of feed was reduced by 0.5 kg/d in the week before farrowing. On the first day after delivery to farrowing crate, the sows were fed 0.5 kg and on the following days, each feed was increased by 0.5 kg until the sows could feed ad libitum. Water was available ad libitum in the trough after feeding. All the sows were fed restrictively with complete feed. Room temperature and humidity ranged from 25┬░C to 32┬░C and 45% to 55%, respectively, during the daytime. During the night, the room temperature varied between 18┬░C and 22┬░C, and the relative humidity was approximately 35%. The barn was ventilated through a natural ventilation system. (The feed contained 12.9 MJ metabolizable energy, 185.0 g crude protein, 50.0 g crude fat, 80.0 g crude ash, and 12.0 g glycine, per kg of feed).
The gestation and farrowing rooms were ventilated by natural ventilation. The temperature of the loose experiment room was 20.9┬░C┬▒1.1┬░C with a relative humidity of 86%┬▒2% during gestation, and room temperature was 18.2┬░C┬▒2.8┬░C, the humidity was 66.5%┬▒3.5% during farrowing and lactation. The confined experiment room temperature was 18.4┬░C┬▒2.2┬░C with a relative humidity of 65.2%┬▒2.3% during gestation, and room temperature was 21.5┬░C┬▒3.2┬░C, the humidity was 80.5%┬▒5.5% during farrowing and lactation. Free drinking and management were the same, as were the daily detection of room temperature and humidity and the regular inspection of the physical condition of all experimental sows. Disinfection was performed once a month.
As the experimental cycle span was long, in view of the impact of ambient illumination on the PLR, it was necessary to distinguish the conditions of the ambient light illuminated by the PLR detection according to different months. The PLR testing time was between 8:30 h to 9:30 h in the morning from November to February and 7:30 h to 8:30 h during March and April. The ambient illumination of the room at this time was 1,621.4┬▒10.1 lx (sunny side) and 801.5┬▒5.7 lx (back sunny side). The pig eye illumination in the gestation crate and parturition hut was 143.1┬▒ 2.6 lx (the dark place of the sunny side), 261.3┬▒8.9 lx (the light place of the sunny side), 40.5┬▒3.6 lx (the dark place of the back sunny side), and 83.5┬▒4.1 lx (the light place of the back sunny side). As the gestation crate was located adjacent to the parturition hut, the intensity of light was basically the same. Therefore, the test period was defined in the morning with illumination meeting the PLR conditions, and the sows at this time were relatively quiet and in a state of lying; thus, the ambient light would not cause stress reactions.
All experimental sows were in good body condition with no apparent signs of illness or lameness at the commencement of the experiment as assessed visually in line with standard farm protocols. Five sows were obtained from different experiment groups on the third day after weaning, the PLR index of every group sow was measured by a new portable pupil detector (NeurOptics PLR-200 Pupillometer, NeurOptics Inc., Irvine, CA, USA). Bao et al [5] found that eye illumination was suitable for the detection of the sowsŌĆÖ pupils under the condition of <250 lx, the pulse intensity was 180 uw, and the pulse duration was 802 ms. The operator kept the tested sowŌĆÖs head from unintentionally shaking so that the lens of the pupil detector remained at the same level as the eye; if the sowŌĆÖs eyes were not open, the operator could skillfully open their eyelid for detection. The measurement was conducted by a single person, and all measurements were taken by the same person throughout the experiment.
The pupillometer operation method was as follows: The user would press the LEFT scan button when detecting the left eye, and press the RIGHT scan button for the right eye. First, the user was required to hold the RIGHT or LEFT scan button down, position the pupillometer on the sowŌĆÖs eye, and center the sowŌĆÖs pupil in the center of the field of view as indicated by a blue rectangle; the pupillometer automatically detected the pupil. The pupil was marked with a green circle drawn around its perimeter. The user released the button when ready to initiate the actual measurement. When the RIGHT or LEFT scan button was released, the actual pupil recording was initiated. The user kept the pupillometer firmly on the sowŌĆÖs eye for the entire duration of the recording, which lasted 5 seconds; the user did not move or remove the device during this time. LAT is the latency of the pupil constriction onset and it represents the time of the onset of the constriction.
Each pig was immediately given a lethal injection of pentobarbital into the ear vein and was transported, deeply anesthetized, to the operating room. Five blood samples were obtained from different experiment groups on the fourth day after weaning, and the samples to measure cortisol concentrations were collected in anticoagulant-free tubes from each sow. The tubes were gently shaken and stored on ice until all animals had been killed. Then, all tubes were centrifuged to extract blood serum. They were kept at room temperature for 1 hour before serum was isolated (centrifugation at 2,000 rounds├Ś10 min at 20┬░C). They were stored at ŌłÆ70┬░C until the assay. The cortisol concentrations were determined using commercial enzyme-linked immunosorbent assay kits (Cortisol Emax Immunoassay System, Invitrogen, Carlsbad, CA, USA).
Each experimental sowsŌĆÖ brain were rapidly excised, the cerebellum was removed, and the brain was transversely cut into left and right hemisphere. The hippocampus, frontal cortex and hypothalamus were located, rapidly dissected and tissue collected. All the samples were snap-frozen in liquid nitrogen, and stored at ŌłÆ70┬░C until further use.
For RNA extraction, equal amount tissues (1 g) were excised in cold RNase-free phosphate buffered saline to process tissue homogenate. The total cellular RNA was extracted from 100 ╬╝L aliquots of the respective tissue homogenate using TRIzol reagent (Invitrogen, Beijing, China) according to the manufacturerŌĆÖs instructions. The RNA was air dried for 2 to 10 min, redissolved in 20 L RNase-free water, and stored at ŌłÆ70┬░C until use. To evaluate RNA quality, the optical density (OD) of RNA at wavelength 260 and 280 nm was examined, respectively. The ratio of OD260 to OD280 was within 1.8 to 2.2 (data not shown). Reverse transcription was carried out in a 40 L reaction mixture containing 20 L RNA using oligo-dT primers. All of the process was conducted under RNasefree conditions. The AMP-specific cDNA was amplified by polymerase chain reaction (PCR) using Ex-Taq polymerase and primers designed internally from three sets of primers, respectively. The PCR protocol was as follows: an initial denaturation for 5 min at 95┬░C followed by 30 cycles of denaturation at 94┬░C for 30 s, annealing at 50┬░C for 30 s, and polymerization at 72┬░C for 1 min. The final polymerization step was performed at 72┬░C for 10 min.
A real-time PCR was performed to determine the content of IL-6 and BDNF cDNA in tissues from sows of both reared in the loose and confined system with the real-time RT PCR method using SYBR Premix EX TaqTM (Takara Biotechnology, Dalian, China). The content of ╬▓-actin was measured to control for variation in RNA yield and RT-reaction conditions. Equal amounts of tissues (1 g) were used to process the tissue homogenate as described above. The total cellular RNAs were extracted, RNA quality was evaluated, and RT was performed as described above. Real-time PCR was performed with the LightCycler 480II Real-Time PCR system (Roche, Switzerland) according to previous studies [14]. T he following primers were used: 18S, forward primer (5ŌĆ▓-GGCTACCACATCCAAGGAAG-3ŌĆ▓), reverse primer (5ŌĆ▓-TCCAAT GGATCCTCGCGGAA-3ŌĆ▓), 149 bp (NR_002170); IL-6, forward primer (5ŌĆ▓-AGATGCCAAAGGTGATGCCA-3ŌĆ▓), reverse primer (5ŌĆ▓-ACAAGACCGGTGGTGATTCTCA-3ŌĆ▓), 257 bp (NM_214 399); BDNF, forward primer (5ŌĆ▓-GTTTCCCTCTGGTCATGGA A-3ŌĆ▓), reverse primer (5ŌĆ▓-GCTGGCGGTTCATAAGGATA-3ŌĆ▓), 251 bp (NM_214259). Mixtures underwent the following real-time PCR protocol: A denaturation program (95┬░C for 10 min) and a three-segment amplification and quantification program (95┬░C for 10 s with a single fluorescence acquisition point, 72┬░C for 15 s) repeated for 40 cycles.
The collected test data are analyzed using the Statistic Package for Social Science (SPSS 23.0; IBM Institute Inc., Chicago, IL, USA). Data are expressed as the mean┬▒standard error of the mean. Effects of restricting stress and age and their interaction were statistically evaluated by analysis of variance with the factors housing (loose vs confined) and age group (young adolescent sows vs full maturity sows). In case of statistical significance, Sidak post hoc comparisons were performed between housing conditions for each age group separately. Statistical significance was set at p<0.05.
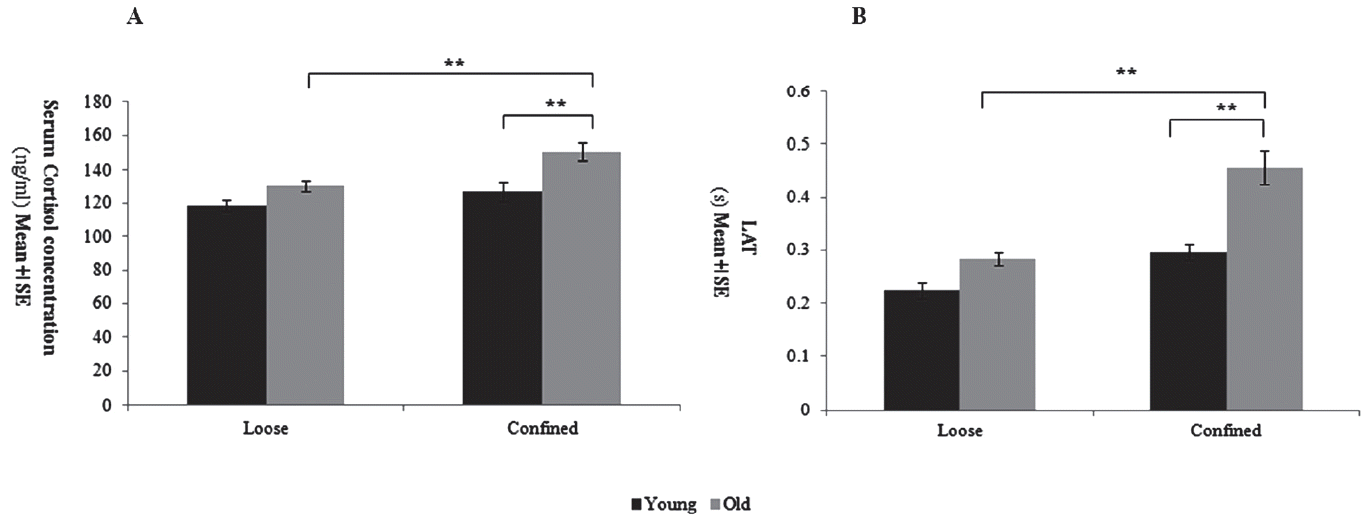
All animals were slaughtered at approximately the same time after weaning of the last litter, i.e. the slaughter interval was not different between the four groups (Young adolescent loose: Young adolescent confined: Full maturity loose: Young adolescent confined = 14.7┬▒2.3:15.1┬▒1.1:15.4┬▒1.6:14.9┬▒2.1, p = 0.541). The full maturity and young adolescent loose sows had similar serum cortisol concentrations (p = 0.233; Figure 2A), but the full maturity confined sows were significant higher than those of the young adolescent confined sows (p<0.05; Figure 2A). Post hoc analysis revealed that serum cortisol concentrations in the full maturity confined sows were significant higher than that in the full maturity loose sows (p<0.05; Figure 2A).
The LAT time of the full maturity sows were significant longer than those of the young adolescent sows (age group: p<0.05; Figure 2B). Housing conditions affected the time of the LAT (housing: p<0.01; housing by age-group interaction: p<0.05; Figure 2B), with full maturity confined sows having significant longer LAT time than young adolescent confined sows or full maturity and young adolescent loose sows (p<0.05; Figure 2B), as confirmed by Sidak post-hoc comparisons.
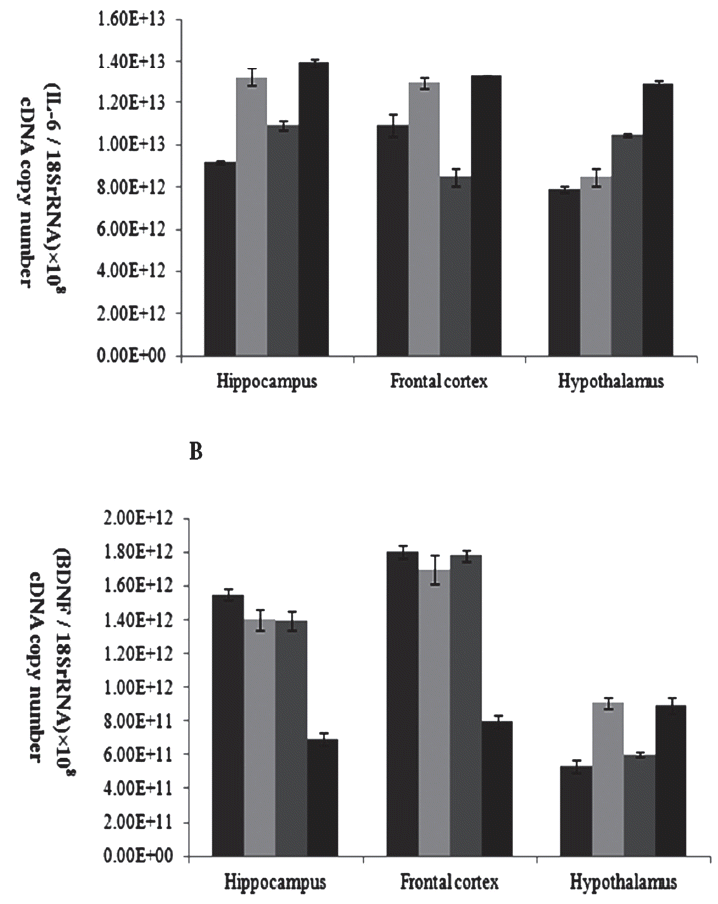
Results indicate that the expression of BDNF mRNA in the hippocampus and frontal cortex sample were higher in the full maturity confined sows than in the other sows (p<0.05; Figure 3A), but this difference was no longer seen in the hypothalamus. While the level of expression of IL-6 mRNA in the hippocampus and frontal cortex in young adolescent and full maturity confined sows was higher than that of the loose sows (p<0.05; Figure 3B), and in the hypothalamus tissue, the level of expression of IL-6 mRNA in full maturity confined sows was higher than young adolescent confined sows or full maturity loose and young adolescent sows (p<0.05; Figure 3B).
The confinement crates during pregnancy stage were a serious source of stress for sows [15], and in this study, we found that the young adolescent loose and confined sows had similar serum cortisol concentrations (Figure 2A). This indicated that the sows could gradually adapt to the stress environment when space-limited stress has not reached a serious or long-term effect. That is, the acute stress-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis is the adaptive response of the animalŌĆÖs body to external stimuli, which would result in short-term excitement of the monoamine neurotransmitter system, such as the glucocorticoids, the locus coeruleus, the raphe nucleus and substantia nigra neurons [16]. The sows would become bored with the restraining environment as the confinement duration increased and would show a failure response pattern characterized by the activation of the HPA system [17]. This was the reason for the significant increase in serum cortisol concentrations in full maturity confined sows, similar to the results of studies by Van der Staay et al, M├Čstl and Palme [3,18]. Throughout the space confinement period, the full maturity sowsŌĆÖ serum cortisol concentrations were higher than those of young sows (Figure 2A), and the results were similar to the findings of Van der Staay et al [3], suggesting that repeated restriction stress can enhance the effect of adverse events on HPA axis activity [19]. It had also been suggested that early confinement experience might improve the release of the glucocorticosteroid levels [20]. Several studies also suggested that since the cortisol could pass through the blood-brain barrier on the HPA axis and affect the regulation of the brainŌĆÖs emotional region [21], the increase in circulating levels was thus associated with depression and stress [22].
On the basis of previous experiments, the PLR indices of the confined and loose sows with different ages at the weaning stage were recorded using the NeurOptics PLR-200 pupillometer (Neur Optics Inc., USA). The results showed that the long-term space constraints had significant effects on the time of LAT in different births. This showed that long-term space constraints had a serious psychological impact on the sows and might lead to the occurrence of depression. In this study, the time of LAT in the full maturity sows was significantly longer than that of other age groups (Figure 2B), which might indicate that the high parity sows that experienced repetitive space constraints were more likely to develop discomfort or that their pupil sensitivity to light stimulation decreased with aging. Bar et al also found that the time of latency in depressive patients was significantly longer than that of healthy subjects [11]. This was consistent with the LAT findings in high parity sows, indicating that long-term and recurrent space constraints might seriously affect the sowsŌĆÖ mental health and produce depression symptoms, causing changes in LAT that are closely related to the pupillary contraction [12,23]. Bitsios et al reported that the increase in latency and the decrease in amplitude and velocity of the PLR in the elderly might be related to increasing age and caused by the decline in the function of the autonomic nerve [24]. In addition, prenatal stress can also cause stress closer to delivery and then weaken the human PLR response [25], significantly lowering the pupillary contraction amplitude [26]. This study found that long-term space constraints led to a significant prolonged latency of the PLR in sows, and the latency of the PLR in high parity sows that had undergone multiple space constraints during gestation was significantly higher than that of young sows (Figure 2B). This was consistent with the phenomenon of prolonged latency of the PLR in sows that had been studied in our earlier experiments [5].
The main finding of this study was that changes in BDNF mRNA levels did not correlate with changes in age and restrict experience which reared in loose condition, but in confined condition the BDNF mRNA levels of full maturity sows were significantly lower than those of young adolescent controls (Figure 3A). In addition, the space-restricting stress during gestation and lactation period might significantly decrease the BDNF mRNA levels of old sows which experienced the recurrent space-restricting stress before, but did not significantly impact primiparous sows as they first experienced space-restricting stress. This matches previous human depression research data [27] that supports the hypothesis of neurotrophic factor deficits in the confined sows that would have a negative correlation of serum BDNF levels with the length of restrict stress. However, we did not find such a correlation between BDNF mRNA levels and ages in the group-housed sows. Some studies reported a negative correlation of serum BDNF levels with the severity of depression [27]. Since the neurotrophic hypothesis of depression is supported by robust evidence of reduced BDNF in patients with depression [28], we consider long-lasting and recurrent space restricting stress on breeding sows had a serious psychological damage, and might be inducing depression disease.
The mechanism by which increased BDNF expression could improve depression is unclear. Duman and colleagues have hypothesized that BDNF induces neuronal sprouting in brain regions like the hippocampus and frontal cortex and improves synaptic connectivity and function of neural circuits involved in mood regulation [28]. The hippocampus is one of several limbic structures that have been implicated in mood disorders. there were reports that stress can cause damage and atrophy of neurons in certain brain structures, most notably the hippocampus, which expresses high levels of receptors for glucocorticoids and disturbed the HPA axis, the major stress reactive adrenal steroid and thereby contribute to other major symptoms of depression [29].
We also found that the expression of IL-6 mRNA in the hippo campus, frontal cortex and hypothalamus sample were higher in the full maturity confined sows than in the other sows (Figure 3B), indicating that space constraints stress had a serious impact on the sowsŌĆÖ psychological condition. Furthermore, the level of expression of IL-6 mRNA in the full maturity confined sows was higher than that of the young adolescent confined sows (Figure 3B), which indicated that long-term and repeated environment constraints would aggravate the sowsŌĆÖ psychological stress. There was also a belief that long-term stress states will have an inhibitory effect on the immune system [30]. In other words, the proinflammatory cytokines could not only activate the HPA axis effectively, but also prevent the negative feedback of corticosteroids to the HPA axis, resulting in persistent hyperactivity of the HPA axis. It had been reported that the negative emotional state of depression can cause a significant increase in IL-6 concentrations [28]. Moreover, the abnormality of HPA axis and the abnormality of cytokine and APP concentrations were the apparent symptoms of chronic stress-induced depression [29].
In this study, we initially measured the variable regularity of sowsŌĆÖ physiological and psychological indexes and cytokine and BDNF gene expression when they were under conditions of confinement stress stimulation. From the results, we observed that the long-term and recurrent space-restraint stress significantly reduced the sowsŌĆÖ latency time of the onset of the pupil constriction and the serum cortisol levels, and it produced a significant changes at the gene-expression level that are consistent with a depression-like symptomatology. We believe that the long-term and repeated confinement stress caused serious psychological trauma to sows, destroying the activity of the HPA axis making sowsŌĆÖ environmental adaptation very poor and causing serious psychological stress.
ACKNOWLEDGMENTS
This study was supported by National Nature Science Foundation of China (31472131) and this study was supported by a grant from the Earmarked Fund for China Agriculture Research System (No. CARS-35). We wish to thank Dr. Jianhong Li. College of Life Science for RNA extraction, reverse transcriptase polymerase chain reaction amplification and the real-time RT-PCR technical assistance.
Figure┬Ā2
Effects of space-restricting stress on serum cortisol concentration (A) and latency time (B) in different age sows, housed either loosely or confined. Young, primiparous sows; Old, 5 parity sows. The data are presented as the mean┬▒standard error of the mean. n = 8 per group, and the data were analyzed using SPSS 23.0. * p<0.05; ** p<0.01.
Figure┬Ā3
Relative concentrations of interleukin-6 (A) and brain derived neurotrophic factor (B) mRNA in the hippocampus, frontal cortex and hypothalamus of different age sows, housed either loosely or confined. The cDNA copy number was measured by real time reverse transcription-polymerase chain reaction and the results were expressed finally for each sample as the copy number of each target cDNA normalized to 108 times the copy number of the reference gene 18S rRNA, using the following formula: (target gene cDNA copy number/18S rRNA cDNA copy number)├Ś108. All assays were performed in four independent experiments with three replicates per experiment, and each bar is the mean┬▒standard deviation.
REFERENCES
1. Marchant JN, Broom DM. Factors affecting posture changing in loose-housed and confined gestating sows. Anim Sci 1996; 63:477ŌĆō85.

2. Aver├│s X, Brossard L, Dourmad JY, et al. Quantitative assessment of the effects of space allowance, group size and floor characteristics on the lying behaviour of growingŌĆōfinishing pigs. Animal 2010; 4:777ŌĆō83.


3. van der Staay FJ, Schuurman T, Hulst M, et al. Effects of chronic stress: A comparison between confined and loose sows. Physiol Behav 2010; 100:154ŌĆō64.


4. Sokolski KN, Nguyen BD, DeMet EM. Decreases in dilated pupil size in depressed patients with age may reflect adrenergic changes. Psychiatry Res 2000; 94:267ŌĆō72.


5. Bao J, Li X, Lv FL, et al. Prolonged latency of pupillary light reflex in confined sows: Possible stress-related symptom? J Vet Behav Clin Appl Res 2013; 8:475ŌĆō8.

6. Grandin T. Effect of rearing environment and environmental enrichment on behavior and neural development in young pigs [PhD Dissertation]. Urbana-Champaign, IL, USA: University of Illinois; 1988.
7. Karl JB, Wolf G, Jochum T, et al. The influence of major depression and its treatment on heart rate variability and pupillary light reflex parameters. J Affect Disord 2004; 82:245ŌĆō52.


8. Fotiou F, Goulas A, Fountoulakis K, Papakostopoulos D. Changes in psychophysiological processing of vision in myasthenia gravis. Int J Psychophysiol 1998; 29:303ŌĆō10.


9. Theofilopoulos N, Mcdade G, Szabadi E, Bradshaw CM. Effects of reboxetine and desipramine on the kinetics of the pupillary light reflex. Br J Clin Pharmacol 1995; 39:251ŌĆō5.



10. Ribeiro AP, Junior DP, Champion T, et al. Effects of topical levobunolol or fixed combination of dorzolamide-timolol or association of dorzolamide-levobunolol on intraocular pressure, pupil size, and heart rate in healthy cats. Arq Bras Med Vet Zoo 2008; 60:1045ŌĆō52.


11. Bar KJ, Greiner W, Jochum T, et al. The influence of major depression and its treatment on heart rate variability and pupillary light reflex parameters. J Affect Disord 2004; 82:245ŌĆō52.


12. Heller PH, Perry F, Jewett DL, Levine JD. Autonomic components of the human pupillary light reflex. Invest Ophthalmol Vis Sci 1990; 31:156ŌĆō62.

13. Erickson K, Drevets W, Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci Biobehav Rev 2003; 27:233ŌĆō46.


14. Ma D, Zhang M, Zhang K, et al. Identification of three novel avian beta-defensins from goose and their significance in the pathogenesis of Salmonella. Mol Immunol 2013; 56:521ŌĆō9.


15. Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacol 1997; 134:319ŌĆō29.

16. Pignatelli D, Maia M, Castro AR, et al. Chronic stress effects on the rat adrenal cortex. Endocr Res 2000; 26:537ŌĆō44.


19. Andres R, Marti O, Armario A. Direct evidence of acute stress-induced facilitation of ACTH response to subsequent stress in rats. Am J Physiol 1999; 277:863ŌĆō8.

20. Morm├©de P, Andanson S, Auperin B, et al. Man Exploration of the hypothalamicŌĆōpituitaryŌĆōadrenal function as a tool to evaluate animal welfare. Physiol Behav 2007; 92:317ŌĆō39.


21. Jo├½ls M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol 2007; 28:72ŌĆō96.


22. Kim JJ, Haller J. Glucocorticoid hyper-and hypofunction: stress effects on cognition and aggression. Ann NY Acad Sci 2007; 1113:291ŌĆō303.



23. Boev AN, Fountas KN, Karampelas I, et al. Quantitative pupillometry: normative data in healthy pediatric volunteers. J Neurosurg 2005; 103:496ŌĆō500.


24. Bitsios P, Szabadi E, Bradshaw CM. The inhibition of the light reflex by the threat of an electric shock: a potential laboratory model of human anxiety. J Psychopharmacol 1996; 10:279ŌĆō87.


25. Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol 1994; 15:321ŌĆō50.


26. Bakes A, Bradshaw CM, Szabadi E. Attenuation of the pupillary light reflex in anxious patients. Br J Clin Pharmacol 1990; 30:377ŌĆō81.



27. Karege F, Perret G, Bondolfi G, et al. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res 2002; 109:143ŌĆō8.


- TOOLS





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





