Genetic correlations between behavioural responses and performance traits in laying hens
Article information
Abstract
Objective
The aim of the study was to evaluate genetic correlations between the behavioural profile and performance in laying hens as an indirect answer to the question whether the observed behavioural responses are associated with increased levels of stress in these birds.
Methods
The assessment of birds’ temperament was carried out using the novel objects test. The behavioural test was conducted in two successive generations comprising 9,483 Rhode Island White (RIW) birds (approx. 4,700 individuals per generation) and 4,326 Rhode Island Red (RIR) birds (approx. 2,100 individuals per generation). Based on the recorded responses, the birds were divided into two groups: a fearful profile (1,418 RIW hens and 580 RIR hens) and a brave/curious profile (8,065 RIW hens and 3,746 RIR hens). The birds were subjected to standard assessment of their performance traits, including SM, age at sexual maturity; ST, shell thickness; SG, egg specific gravity; EW, mean egg weight; IP, initial egg production; and HC, number of hatched chicks. The pedigree was three generations deep (including two behaviour-recorded generations). Estimation of the (co)variance components was performed with the Gibbs sampling method, which accounts for the discrete character of the behavioural profile denotation.
Results
The analyses revealed negative correlations between the performance traits of the laying hens and the behavioural profile defined as fearful. In the group of fearful RIW birds, delayed sexual maturation (0.22) as well as a decrease in the initial egg production (−0.30), egg weight (−0.54), egg specific gravity (−0.331), shell thickness (−0.11), and the number of hatched chicks (−0.24) could be expected. These correlations were less pronounced in the RIR breed, in which the fearful birds exhibited a decline in hatchability (−0.37), egg specific gravity (−0.11), and the number of hatched chicks (−0.18). There were no correlations in the case of the other traits or they were positive but exhibited a substantial standard error, as for the egg weight.
Conclusion
To sum up the results obtained, it can be noted that behavioural responses indicating fearfulness, i.e. escape, avoidance, and approach-avoidance may reflect negative emotions experienced by birds. The negative correlations with performance in the group of fearful hens may indirectly indicate a high level of stress in these birds, especially in the white-feathered birds, where stronger performance-fearfulness correlations were found. Fearful birds should be eliminated from breeding by inclusion of the behavioural profile in the selection criterion in the case of laying hens.
INTRODUCTION
Through evolution, hens have developed a low threshold of excitability, high alertness, and quick response to possible threats as a defence mechanism. However, excessive fearfulness is a negative emotional state and, as one of the factors causing corticosterone secretion, may lead to a number of disorders [1]. Prolonged stress negatively influences the function of the entire organism, resulting in reduced birds’ performance, deterioration of their welfare, and behavioural abnormalities [2–7]. Therefore, it seems necessary to lower the degree of fearfulness and the level of stress in breeding birds.
Fear in hens is most frequently measured with behavioural tests, in which the birds are exposed to novel situations (open-field tests) or shown sudden novel stimuli (NOT test) [8–9]. However, it is difficult to determine unambiguously whether the observed responses, which can be defined as fearful behaviour, indeed reflect the emotion of fear [10] and, first of all, whether the hypothalamic-pituitary-adrenal axis (HPA) increasing glucocorticoid secretion is activated in such circumstances [11]. It is evident that the behavioural response to a specific stimulus can be analogous to a stressful situation experienced by the bird and a situation when the HPA axis is not activated [11]. Simultaneously, many types of birds’ behaviour observed in behavioural tests cannot be easily assigned to a particular emotion, as they are ambiguous and indicate motivational conflict. Yet, based on the assumption that excessive stress deteriorates performance [12–14], a hypothesis can be proposed that the behavioural profiles of hens characterised by increased susceptibility to stress should be negatively correlated with performance traits, unlike in birds with low levels of corticosterone secretion.
The aim of the study was to evaluate the genetic relationships between the behavioural profile of laying hens and their performance as an indirect answer to the question whether the behavioural responses are associated with increased levels of stress in these birds.
MATERIALS AND METHODS
Characteristics of the stock
The investigations were conducted on a layer-breeding farm. The analyses were performed in two hen lines: Rhode Island White (RIW), i.e. white-feathered birds, and Rhode Island Red (RIR), which are red-feathered birds. The birds were caged separately in a windowless, artificially lit building equipped with a mechanical ventilation system. The cages were equipped with nipple drinkers and a mechanical feed delivery system. The hens received veterinary supervision.
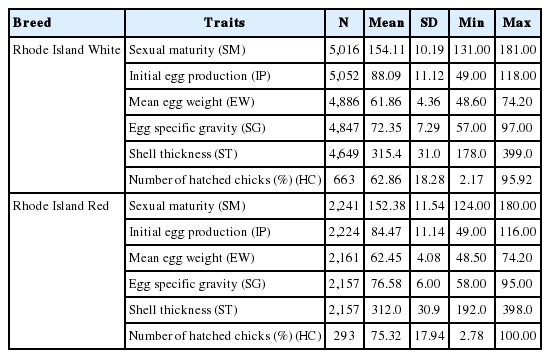
Each hen on the farm was assessed separately in terms of performance traits. They comprised the age at sexual maturity recorded at the time of the first egg laying (age at sexual maturity, SM), mean collective egg weight at week 34 of layers’ life (initial egg production, IP), initial egg production, i.e. the number of eggs laid over the first 15 weeks of egg laying (IP), egg specific gravity calculated according to Archimedes’ principle from egg weight and egg weight in water (egg specific gravity, SG), destructive shell thickness measured in the middle of the egg longitudinal axis with the use of a micrometric screw (shell thickness, ST), and the number of hatched chicks from eggs laid during the laying period (number of hatched chicks, HC) [15]. The number of hatched chicks was controlled in the reproductive stock comprising birds chosen after evaluation of their breeding value and selection. The level of the performance traits in the population is presented in Table 1.
The mating system employed on the farm minimised the level of relatedness between the individuals. The maximum inbreeding of consecutive generations was 15% in only 0.18% of the entire population, whereas the inbreeding rate of 99.5% of individuals did not exceed 5%. In each season, the reproduction stock was formed of 12 hens and a cock. Hen insemination was applied and hatch eggs were collected for 14 consecutive days.
Behavioural test
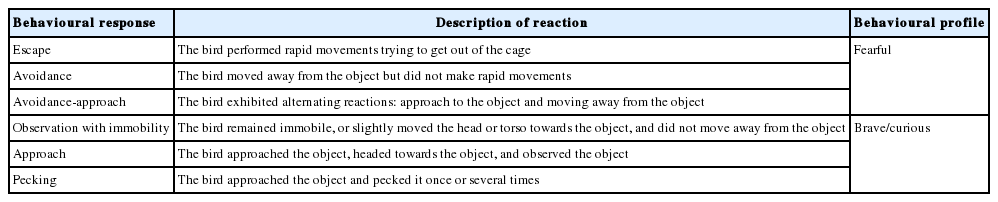
Birds’ temperament was evaluated with the novel object test (NOT). This test was chosen, as its course resembles everyday situations facing birds, e.g. reading out their performance using a laser reader; therefore, it was assumed to characterise well hens’ reactions in the breeding environment. A shiny pencil was moved at a distance of 1 cm from behind the cage wall towards the hen and held still for 30 s. The test was carried out on hens located every four cages so that birds from neighbouring cages had no possibility to see the object beforehand. The behavioural test was performed in two successive generations and the behaviour of 9,483 RIW hens (approx. 4,700 individuals per generation) and 4,326 RIR birds (approx. 2,100 individuals per generation) was analysed. A single recording of birds’ behaviour was carried out; next, the films were analysed and each element of the behaviour was specified. In the pilot research, the test had been applied 4 times at one-week intervals on a randomly chosen population of 500 RIW individuals. Thus, the determined reproducibility of hens’ responses turned out to be high, i.e. ca. 0.8. Therefore, a single test was applied in this study, bearing in mind that repetition thereof in such a great number of birds would have been hardly possible.
Based on the recorded responses, the birds were divided into two groups: a fearful profile (1,418 RIW hens and 580 RIR hens) and a brave/curious profile (8,065 RIW hens and 3,746 RIR hens). A detailed description is presented in Table 2. The division into the two profiles was based on the assumption that animals’ behavioural responses provide indirect information about the emotions that they experience [16].
Statistical analyses
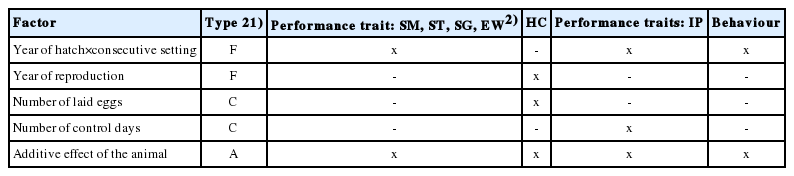
Models for estimation of variance and covariance were developed based on the significance of the fixed environmental effects, which were first verified with the analysis of variance for fixed models (GLM - General Linear Model procedure). Factors included in the mathematical models are presented in Table 3.
The year of hatch is the year of layer’s hatching and concurrently denotes the successive generation. In turn, the consecutive setting denotes the setting from which the layer originates. Birds can originate from four settings carried out at one-week intervals. Hence, the age difference between the oldest and youngest layer in one generation can be 4 weeks. The year of reproduction denotes the year in which eggs assigned for hatching were collected from the laying hen. The number of control days denotes the number of days on which the egg laying performance was assessed in each hen.
The pedigree was three generations deep (including two behaviour-recorded generations). Estimation of the (co)variance components was performed with the THRGIBBS1F90 software [17], which accounts for the discrete character of the behavioural profile denotation. Three hundred thousand samples were obtained with 100,000 discarded as burn-in, following graphical inspection of the posterior chain, inspection of the effective sample size of the parameter of interest as well as checking of the diagonal of the error variance whose all elements mixed/converged to unity.
RESULTS AND DISCUSSION
Like all other animals, birds continually respond to environmental stimuli. The responses to these stimuli are determined by specific characteristics of each bird and may differ significantly among the individuals [18–20]. The profile of behavioural responses depends on birds’ living habitat, previous experience, environmental conditions prevailing during embryonic development, epigenetic effects, and genetic determinants [21,22]. The different behavioural reactions and the different modes of coping with different situations are associated with animals’ previous experience on the one hand and genetic determinants on the other [2]. It has been shown in the present study that additive effects determined the behavioural profile in the range of 0.08 to 0.19, depending on the line (fearful: 0.19/0.08, curious: 0.17/0.19), which creates a possibility of inclusion of behaviour to the selection criterion in laying hens (in print).
In this study, we have verified whether the defined behavioural profiles are related to the performance level. As shown in numerous investigations, prolonged or frequent stress exerts a negative effect on the organism, leading to a number of disorders, e.g. reduced productivity [2–4]. Birds with a low level of corticosterone response can achieve better productivity results, as they exhibit an earlier onset of maturation [23,24]. Therefore, the level of performance traits in hens showing different behavioural responses can indirectly provide information whether the observed responses are associated with elevated levels of stress.
The present investigations have shown negative correlations between the performance traits and the behavioural profile referred to as fearful (Table 4). In the group of fearful birds from the RIW line, delayed maturation as well as reduced initial egg production, egg weight, egg specific gravity, shell thickness, and the number of hatched chicks could be expected. These correlations were less pronounced in the fearful birds from the RIR line, in which decreased egg production, specific gravity, and number of hatched chicks could be expected. In the case of the other traits, there were no correlations, or the correlations were positive but exhibited a substantial standard error, as for the egg weight. The results obtained, which differed between the hen lines, may suggest that the fearful RIW birds experience greater stress in breeding circumstances than the RIR birds. On the one hand, this confirms the fact that despite analogous behavioural reactions, corticosterone responses may vary greatly even between individuals and between breeds [1]; on the other hand, it has been shown that white-feathered hens are more susceptible to stress than hens with dark feathers [2,12,14,25].
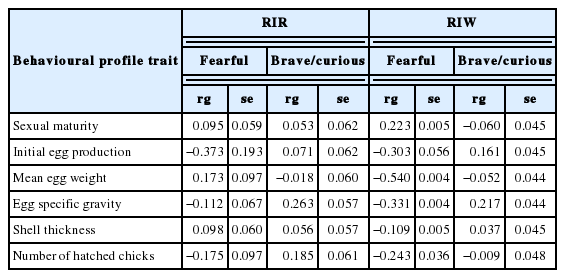
Genetic correlations (rg) and their standard errors (se) between the performance traits and behaviour of RIR and RIW hens
The analysis of the genetic correlations between the performance traits and the brave/curious behavioural profile did not reveal such clear relationships in the RIR and RIW lines. Nevertheless, positive correlations were found for egg specific gravity in both lines and for the number of hatched chicks in RIR and initial egg production in RIW. The absence of such unambiguous correlations, exclusively negative in the fearful birds and positive in the brave/curious hens, suggests that these relationships are not a result of a common genetic background for performance and behavioural traits, but they are rather an effect of the exposure of the fearful birds to stress, especially in the RIW line.
Hens representing the “fearful” behavioural profile can be counterparts of reactive birds. The mode of coping with various situations is defined as animal’s temperament [26] and personality [27]. As demonstrated, the reactive personality is related to relatively strong corticosterone responses, and such birds are characterised by passive timid and slow behavioural responses [1]. Birds defined as fearful exhibited reactions suggesting indecision and internal conflict, e.g. a simultaneous attempt to approach and escape from the object. Reactions indicating motivational conflict undoubtedly contribute to increased secretion of stress hormones, as the animal must make a quick decision how to qualify a given object but is not able to classify the stimulus appropriately to the appetitive or aversive group. Birds with the brave/curious profile can be defined as corresponding to pro-active personality. Birds with this personality exhibit relatively bold and rapid behavioural responses and relatively weak corticosterone responses to stress stimuli [1].
The presented results indicate that selection of birds should take into account the behavioural profile in order to eliminate fearful individuals. This is caused not only by the reduced performance and reproduction traits in these birds but also by the fact that hormones released in stress situations can be deposited in hatch eggs, which consequently leads to behavioural anomalies in the offspring [28,29]. The behavioural profile should be another criterion included in the selection index of laying hens.
To sum up the results, it can be claimed that the behavioural responses indicating fearfulness, i.e. escape, avoidance, and approach-avoidance, can be indicators of negative emotions experienced by birds. Negative genetic correlations have been shown between this type of hens’ behaviour and their performance traits, which may indirectly evidence the high level of stress in these birds, in particular in white-feathered hens, in which stronger performance-fearfulness relationships were noted.
Fearful birds should be eliminated from breeding upon inclusion of the behavioural profile into the selection criterion in laying hens, and responses that define fearfulness, i.e. escape and avoidance of the test object, can serve as indicators.
ACKNOWLEDGMENTS
Supported by the National Centre for Research and Development, Poland. Grant no. PBS2/B8/8/2013.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.