Impacts of post-mortem ageing prior to freezing on technological and oxidative properties of coarse ground lamb sausage in a model system
Article information
Abstract
Objective
The objective of this study was to evaluate the effects of ageing time of lamb loins prior to freezing on technological characteristics and oxidation stability of coarse ground lamb loin sausage using in a model system.
Methods
Lamb loins (M. longissimus lumborum, n = 25) were aged at −1.5°C for 0, 1, 2, 3, and 8 wk and then frozen for the remaining days (a total of 30 wk). The aged/frozen/thawed lamb loins were ground, and model sausages were formulated with 75% aged/frozen/thawed lamb loin, 25% water, 1.5% sodium chloride (NaCl) and 0.3% sodium tripolyphosphate. The pH and thaw/purge loss of aged/frozen/thawed lamb loins were evaluated, and protein functionality (protein solubility and emulsifying capacity), water-holding capacity and textural properties of model sausages were determined. Cooked model sausages were vacuum-packaged in a plastic bag and displayed under continuous fluorescent natural white light (3°C±1°C). Colour and lipid oxidation of the cooked model sausages were evaluated on 0 and 21 d of display storage.
Results
Ageing prior to freezing had no impact on pH and purge/thaw loss of lamb loins and the colour of cooked sausages (p>0.05) made from the loins. Lamb loins aged for at least 3 wk prior to freezing numerically improved total and myofibrillar protein solubilities (p>0.05) and emulsion activity index (p = 0.009) of meat batter, but decreased cooking loss (p = 0.003) and lipid oxidation (p<0.05) of model sausages.
Conclusion
This study suggests that post-mortem ageing of raw meat prior to freezing could improve water-holding capacity and lipid oxidative stability of sausage made from the meat.
INTRODUCTION
Post-mortem ageing has a positive impact on eating quality attributes of meat, such as tenderness, flavour and/or juiciness [1]. Along with such advantages to the palatability attributes, several recent studies found that an adequate post-mortem ageing prior to freezing could minimize the quality gaps between fresh-never-frozen and frozen/thawed meat, in relation to colour stability, water-holding capacity (WHC) and/or tenderness [2–7]. While post-mortem ageing is mostly practiced for fresh meat (carcasses, primals, sub-primals, and/or steaks) to improve eating quality as stated above, an improvement in the protein functionalities of meat during certain time of post-mortem storage has been also reported [8]. Farouk and Wieliczko [8] suggested that proteolysis induced by endogenous enzymes could improve protein solubility, but extended post-mortem ageing could accumulate oxidized products of fat and protein, thus decreasing protein functionality.
Muscle proteins, which are largely categorized into myofibrillar, sarcoplasmic, and stromal proteins, play important roles in determining the quality attributes of processed meat products. In particular, myofibrillar protein primarily contributes to technological properties of processed meat, such as WHC, emulsification, thermal gelation and texture. Sarcoplasmic protein, mainly myoglobin, is responsible for colour development and stability [9]. In practice, most commercial processed meat products are manufactured using frozen/thawed meat, frozen at undetermined post-rigor time. However, it is well documented that freezing/thawing process could reduce the functionality of myofibrillar protein [10] and inversely affect the colour and oxidative stabilities of processed meat products [11]. However, there has been little information on the post-mortem ageing effects of frozen/thawed meat on protein functionality and oxidation stability of further processed meat products.
Taken together, it would be reasonable to hypothesize that since post-mortem ageing can enhance the protein functionality of meat and minimize freezing/thawing related quality defects, manufacturing processed meat products using aged then frozen/thawed meat can result in better quality attributes compared to the products using frozen/thawed meat without post-mortem ageing. Therefore, the objective of this study was to evaluate the effects of different ageing periods (0, 1, 2, 3, and 8 wk) of lamb loins prior to freezing on technological properties and oxidative stability in a model sausage system.
MATERIALS AND METHODS
Raw meat preparation and ageing/freezing/thawing procedure
Twenty-five lamb loins (M. longissimus lumborum) were obtained from one side of 25 male lamb carcasses (approximate age at slaughter: 8 mo, live weight: 44 kg) at 2 d post-mortem, weighed, and individually vacuum-packaged. Lamb loins (five loins per each treatment) were randomly assigned to five different ageing periods (at −1.5°C) prior to freezing (30 wk of total ageing/freezing period): i) frozen for 29 wk and 5 d at 2 d post-mortem (non-aged control), ii) aged 1 wk then frozen for 29 wk (1A), iii) aged 2 wk then frozen for 28 wk (2A), iv) aged 3 wk and frozen for 27 wk (3A), and v) aged 8 wk and then frozen for 22 wk (8A).
After each assigned ageing period, the aged lamb loins were immediately frozen in a conventional freezer (−18°C) for each remaining period. The aged and frozen lamb loins were thawed in a 3°C±1°C cooler overnight, blotted with a paper towel, and then, the weight and pH of the thawed samples were measured. The thawed loins were trimmed of subcutaneous and seam fat and visible connective tissue, and then cut into sub-sections and randomly assigned to different batches.
Model sausage manufacture
Five model sausage treatments were prepared with control, 1A, 2A, 3A, and 8A lamb loins, respectively (550 g/treatment/batch), and the sausage manufacture for both two batches was performed in the same day. The model sausages were formulated with 75% aged/frozen/thawed lamb loin and 25% cold water, and 1.5% sodium chloride (NaCl) and 0.3% sodium tripolyphosphate added based on the total weight of loin and water. The aged/frozen/thawed lamb loins were ground through an 8-mm plate using a meat grinder (MEW 603D, Maschinenfabrik Dornhan, Baden-Württemberg, Germany), and then the ground lamb loins were mixed with cold water and NaCl for 3 min using a mixer (A200, Hobart corporation, Troy, OH, USA). The sodium tripolyphosphate was added and further mixed for 2 min, and the meat batter was kept stationary for 2 min in the mixer. To uniformly mix all ingredients and additives, the mixture was re-ground through a 3-mm plate using the meat grinder. The temperature of the mixture was maintained below 10°C during manufacturing process, monitored with a digital temperature logger (OctTemp2000, MadgeTech, Inc., Warner, NH, USA) with a thermocouple (T-type, Omega Engineering, Stamford, CT, USA).
The meat batter (40 g) was stuffed into a 50 mL centrifuge tube (6 tubes/treatment/batch) by using a stuffer, and the samples were centrifuged at 900×g for 15 min at 4°C. The stuffed meat batters were cooked in a 75°C water bath for 30 min until the core temperature reached to 72°C, monitored with a Digi-Sense scanning temperature logger (Eutech Instruments Pte Ltd., Singapore, Singapore). The cooked samples were immediately placed in an ice-water slurry to cool down the temperature to below 10°C. Three cooked model sausages from the six tubes per treatment were placed in a plastic bag (oxygen permeability 5 cc/m2/24 h at 23°C and 0% relative humidity, D&L Marketing International Ltd., Auckland, New Zealand), vacuum-packaged, and displayed for 21 d in a 3°C±1°C cooler under continuous fluorescent natural white light (1,350 lx, color rendering index = 82, colour temperature = 4,000 K; Osram, Auckland, New Zealand). On 0 and 21 d of display storage, the colour and lipid oxidation of the cooked model sausages were evaluated.
Physical and chemical measurement
pH measurement: The pH of lamb loins (n = 5/treatment) was measured in duplicate by inserting an electronic pH-meter equipped with a probe (Hanna 99163 pH meter with a FC232D combined pH/temperature probe, Hanna Instruments, Woonsocket, RI, USA).
Purge/thaw loss: Purge/thaw loss of aged and frozen/thawed lamb loins (n = 5/treatment) was determined by calculating the differences in weight before freezing and after thawing expressed as a percentage.
Protein solubility: Protein solubility (total, myofibrillar, and sarcoplasmic proteins) of meat batter (n = 2/treatment/batch) was determined in duplicate as described in Farouk and Swan [12]. To determine the solubility of total proteins, two grams of meat batter were weighed into a centrifuge tube and homogenized with 20 mL of cold 1.1 M potassium iodide in 0.1 M phosphate buffer (pH 7.4) using an Ultra-Turrax (Lab Supply Pierce, Auckland, New Zealand) at 13,500 rpm for 20 s. The suspension was centrifuged at 6,000×g for 15 min (4°C) and filtered with filter paper (Whatman No. 1). The protein concentration of supernatant was determined according to Biuret method [13]. To determine the solubility of sarcoplasmic proteins, meat batter was treated with the same extraction procedure above, using 0.025 M phosphate buffer (pH 7.4). The solubility of myofibrillar proteins was determined by calculating the difference between total and sarcoplasmic proteins, and the protein solubility was expressed as % extractable proteins.
Emulsifying capacity: The emulsifying capacity (emulsion activity index [EAI] and emulsion stability [ES]) of extracted protein from meat batters (n = 2/treatment/batch) was according to the procedure of Pearce and Kinsella [14]. The protein concentration of the total soluble proteins from the protein solubility assay was adjusted to 400 mg/mL using distilled water. Five millilitres of the protein solution and 2 mL of safflower oil were homogenized using the Ultra-Turrax at 20,500 rpm for 1 min. Fifteen microlitres of the emulsified layer was collected and immediately vortex-mixed with 3 mL of 0.3% sodium dodecyl sulfate. The absorbance of the mixture was read at 500 nm (Biotek Epoch, Biotek Inc., Winooski, VT, USA). After the absorbance reading, the samples were kept stationary at room temperature for 2 h, and the absorbance of the samples was read again at 500 nm. The ES of extractable protein was determined by calculating the difference between the initial absorbance and the absorbance after 2 h.
Expressible moisture: An 1.5±0.3 g of meat batter (n = 2/treatment/batch) was weighed in a 50 mL centrifuge tube, wrapped with filter paper (Whatman No. 4), and then centrifuged at 1,000× g for 15 min at 4°C [15]. Expressible moisture was calculated as follows; expressible moisture (%) = (weight of expressed water in filter paper [g]/weight of meat batter [g])×100.
Cooking loss: The cooking loss of model sausages (n = 3/treatment/batch) was calculated as follows; cooking loss (%) = ([the weight of raw sample {g} – the weight of cooked sample {g}]/the weight of raw sample [g])×100.
Texture profile analysis: Textural properties of the cooked sausages (n = 3/treatment/batch) were performed by the method of Bourne [16] using a texture analyser (TA; HT plus, Stable Micro Systems, Haslemere, England). The cooked samples used for cooking loss determination were cut into uniform size (12.5 mm diameter×10 mm length). The samples were compressed twice to 75% of the original height with an aluminium cylinder probe of 2.5 cm diameter and a 50 kg load cell at a cross speed of 5 mm/s. Data were collected and analysed for hardness (N), springiness, cohesiveness, gumminess (N), and chewiness (N).
Instrumental colour evaluation: Surface colour (Commission Internationale de l’Eclairage [CIE] L*, a*, and b*) on cross-section of cooked sausages (n = 3/treatment/batch) was measured using a Minolta Colour Meter (Illuminant D65, 1 cm diameter aperture, 10° standard observer; CR-300; Konica Minolta Photo Imageing Inc., Tokyo, Japan). Calibration was performed using a standard white, and CIE L*, a*, and b* values were recorded (four readings per sample). Hue angle was calculated as follows; hue angle = tan−1(b*/a*) [17].
2-Thiobarbituric acid reactive substances: 2-Thiobarbituric acid reactive substances (TBARS) value of cooked sausages (n = 2/treatment/batch) was determined according to the method of Buege and Aust [18] with minor modifications. Six milliliters of distilled water were added to 2 g of sample prior to homogenization (IKA Ultra-Turrax T25, IKA, Freiburg, Germany) at 15,000 rpm for 15 s. All samples were centrifuged at 2,000×g for 10 min at 4°C, and 2 mL of supernatant was added to a tube containing 4 mL of trichloroacetic acid (TCA)/2-thiobarbituric acid (TBA) reagent (20 mM TBA in 15% TCA solution). The tubes were heated in a 95°C water bath (VWR 1211, VWR Sci., Radnor, PA, USA) for 15 min and cooled to ice water. The samples were centrifuged at 2,000×g for 10 min at 25°C and then filtered using filter paper (Whatman No. 4). The absorbance was measured at 531 nm (Biotek Epoch, Biotek Inc., USA), against a blank prepared with distilled water (2 mL) and TCA/TBA reagent (4 mL). The TBARS value was calculated using a molecular extinction coefficient (1.56×105 M−1 cm−1) and expressed mg malonaldehyde per kg of sample (mg MDA/kg sample).
Statistical analysis
Experimental design was a completely randomized design, and data were analysed using analysis of variance procedure of GenStat (12th Edition, 2010; GenStat for Windows, version 12.20.3717, VSN International, Oxford, UK). Animal and batch were used as a block for lamb loins and model sausages, respectively, and treatment (different ageing time prior to freezing) and storage time (only for colour and TBARS data) were analysed as fixed effects. For TBARS analysis, the restricted maximum likelihood (REML) was used as there were unequal replications within the batches. For the REML model, batch was included as a random effect and treatment and storage time as fixed effects. An interaction between storage periods (0 and 21 d) and treatment was included as fixed effect for colour and lipid oxidation analysis.
RESULTS AND DISCUSSION
pH and purge/thaw loss of aged/frozen/thawed lamb loins
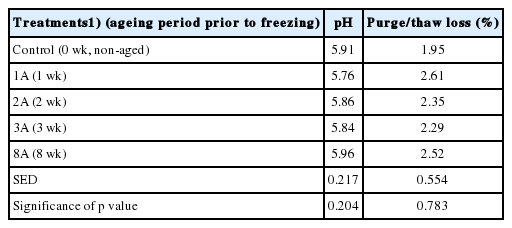
Effects of ageing period prior to freezing on pH and purge/thaw loss of lamb loins are shown in Table 1. The pH of lamb loins numerically but not significantly increased from 5.76 to 5.96 with extended ageing. Similar outcomes were previously reported [4,5]. The purge/thaw loss of aged/frozen/thawed lamb loins was unaffected by ageing period prior to freezing (1.95% to 2.61%; p>0.05). Therefore, it would be reasonable to state that pH and initial moisture loss of aged/frozen/thawed lamb loins would not be attributing factors affecting the quality characteristics of model sausages manufactured from the loins.
Protein functionality of meat batter from aged/frozen/thawed lamb loins
The functionality of muscle proteins, such as solubility, emulsifying capacity, and gelling ability, is the most important factor affecting WHC and textural properties of restructured meat products [8,9,19,20]. In particular, salt-soluble proteins (mainly myofibrillar proteins) in a raw meat ingredient is responsible for the formation of a stable gel matrix comprising insoluble fat and other components [19].
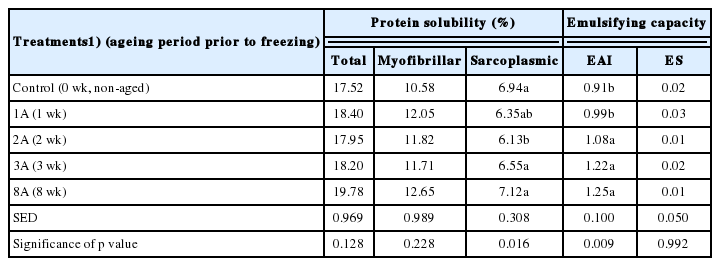
The effects of ageing prior to freezing on the protein function ality (solubility and emulsifying capacity) of meat batters are shown in Table 2. Although not statistically different, the meat batter formulated with aged/frozen/thawed lamb loins (1A, 2A, 3A, and 8A treatments) exhibited numerically higher solubilities of total and myofibrillar proteins compared to that prepared with frozen/thawed only lamb loins (control). On the other hand, the solubility of sarcoplasmic proteins decreased up to 2 wk (p<0.05), and then slightly increased. According to Farouk and Wieliczko [8], the solubilities of total and myofibrillar proteins of beef muscle increased with post-mortem storage up to 3 wk, whereas the solubility of sarcoplasmic proteins decreased. Our results indicate that the changes in protein solubilities with extending ageing period prior to freezing were consistent with the previous findings [8]. Regarding the changes in protein solubilities during post-mortem storage, Farouk and Wieliczko [8] suggested that the increased total and myofibrillar protein solubilities could be attributed to the generation of soluble peptides and amino acids from muscle proteins by post-mortem proteolysis.
Protein solubility and emulsifying capacity of meat batter formulated with aged/frozen/thawed lamb loins
The EAI of extractable protein fraction from meat batter pre pared with aged and frozen/thawed lamb loins steadily increased with extending ageing periods prior to freezing (p<0.05). In particular, ageing for at least 2 wk prior to freezing (2A, 3A, and 8A treatments) resulted in significantly higher EAI of protein fraction from meat batter compared to non-aged control. However, ES of protein fraction from meat batter was unaffected by ageing period of lamb loins prior to freezing (p>0.05). Numerous previous studies have indicated that freezing/thawing causes the decline of protein functionality due to a decrease in protein solubility, which in turn results in inadequate technological properties of restructured meat products [10,20–22]. In this regard, it is of interest to note that an increase in ageing periods of lamb loins prior to freezing increased EAI of protein fraction from meat batter prepared with frozen/thawed lamb loins. According to Farouk and Wieliczko [8], an increase in soluble protein during post-mortem storage could contribute to the formation of stable cross-linkages to entrap more moisture and fat. In this current study, thus, the improved protein functionality of model sausages formulated with aged and frozen/thawed lamb loins might be associated with the impact of ageing prior to freezing.
WHC of model sausages from aged/frozen/thawed lamb loins
The effects of ageing of lamb loins prior to freezing on WHC (expressible moisture and cooking loss) of model sausages are shown in Table 3. Although it was not significant, model sausages formulated with lamb loins aged for 2, 3, and 8 wk prior to freezing (2A, 3A, and 8A treatments) showed numerically lower expressible moisture losses than control. Moreover, the sausages formulated with lamb loins aged for 3 and 8 wk prior to freezing (3A and 8A treatments) exhibited a significantly lower cooking loss than model sausage made with control (non-aged lamb loins). Similar trends have been reported by previous studies that extended post-mortem ageing could improve WHC of frozen/thawed meat, particularly indicated by a decrease in purge and/or drip loss [2,3,5–7]. In general, the thermal gelation of soluble muscle proteins during heating process is greatly responsible for cooking loss/yield of restructured meat products [23]. According to Farouk and Wieliczko [8], an increased cooking yield of beef muscles was found with post-mortem storage for 2 wk, due to the improvement of gelling ability with increasing amount of soluble proteins. In this current study, the reduced cooking loss of model sausages prepared with aged/frozen/thawed meat might be partially attributed to the higher solubilities of total and myofibrillar proteins and emulsifying capacity compared to non-aged/frozen/thawed lamb loins. Further studies determining underlying mechanisms on the positive effect of post-mortem ageing prior to freezing on WHC of processed meat products would be highly warranted.
Textural properties of model sausages from aged/frozen/thawed lamb loins
The effects of ageing of lamb loins prior to freezing on textural properties of cooked model sausages are shown in Table 4. Hardness, gumminess, and chewiness of model sausages were affected by ageing period of lamb loins prior to freezing (p<0.05), however, there was no significant differences in springiness and cohesiveness. Ageing of lamb loins for 8 wk prior to freezing (8A) resulted in lower hardness, gumminess, and chewiness of model sausages compared to control sausage (p<0.05), which indicates that an extended ageing period of initial raw meat prior to freezing could result in more soft texture of further processed meat products. In general, hardness and springiness are representative parameters evaluating the textural properties of restructured meat products [24]. Hardness, which is a primary parameter determining second parameters (gumminess and chewiness), is affected by water and/or fat release from meat batter during heating, subsequently affecting dry matter of cooked final products [24,25]. Thus, our finding for the soft texture (decreased hardness) of the sausages could be associated with the decreased cooking loss, leading to the increased moisture content in the cooked sausage.
Colour of cooked model sausages from aged/frozen/thawed lamb loins
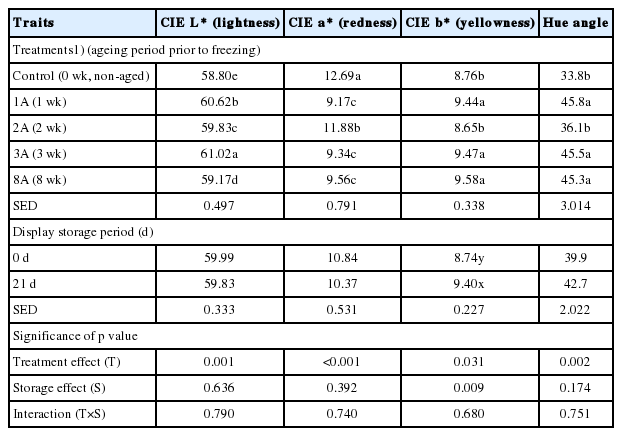
The effects of ageing of lamb loins prior to freezing on colour characteristics of cooked model sausages on 0 and 21 d of display storage are shown in Table 5. No significant interactions between treatment and storage effects were found on all colour parameters, including CIE L* (lightness), a* (redness), b* (yellowness), and hue angle (discolouration). While there were significant differences in lightness (58.80 to 61.02), redness (9.17 to 12.69), and yellowness (8.65 to 9.58) of model sausages, the small differences between the colour parameters of treatments would not be practically meaningful to make a visual difference. However, the sausages formulated with lamb loins aged for 1, 3, and 8 wk prior to freezing (1A, 3A, and 8A treatments) had a significantly higher hue angle than the sausage prepared with control lamb loins. This observation would be likely due to the decreased a* and the increased b* values of the loins with further ageing. In general, a decrease in redness of fresh meat during storage could result from metmyoglobin accumulation. According to King and Whyte [26], metmyoglobin is highly sensitive to heat denaturation, which forms hemichromogen (brown colour) after cooking. Thus, the decreased redness and increased hue angle of cooked model sausages prepared with aged/frozen/thawed lamb loins might be related to high proportion of metmyoglobin with extended ageing time. During display storage, the yellowness of sausages slightly increased (p<0.05). However, there were no significant differences in lightness, redness, and hue angle of model sausages between 0 and 21 d of display storage. Thus, our results indicate that the extended ageing period of meat prior to freezing resulted in initially different colour characteristics of cooked model sausages, but no adverse impacts on colour stability.
Lipid oxidation of cooked model sausages from aged/frozen/thawed lamb loins
Lipid oxidation of cooked sausages was affected by both the main effects, ageing prior to freezing treatment and display storage period (p<0.05; Figure 1). However, no significant interaction of those two main effects was found. On 0 day of storage immediately after cooking, an increase in post-mortem ageing period of lamb loins prior to freezing decreased TBARS value of cooked sausages (p<0.05). In particular, the TBARS value of cooked sausages formulated with lamb loin aged for 8 week prior to freezing (8A treatment) was significantly lower than that of cooked sausages prepared with control (non-aged). On 21 d of display storage, the TBARS value of all cooked sausages substantially increased (p<0.05). However, cooked sausages made with lamb loins aged for 3 and 8 wk prior to freezing (3A and 8A treatments) had still lower TBARS values than control sausage (p<0.05).

2-Thiobarbituric acid reactive substances (TBARS) value of cooked model sausages formulated with aged/frozen/thawed lamb loins on 0 and 21 d of display storage. Treatments: lamb loins were collected at 2 d post-mortem, aged (0, 1, 2, 3, and 8 wk) and frozen for each remaining period, respectively. The total period for the ageing and freezing process was equally 30 wk. a,b Means within different letters in each storage period indicate differences between treatments (p<0.05).
In addition, the TBARS values of the cooked sausages were around 0.5 mg MDA/kg, which was suggested as the threshold of rancid flavours and odours by trained sensory panels due to lipid oxidation [27]. Previously, inconsistent results on the effects of ageing on lipid oxidation of fresh (or frozen/thawed) meat have been reported. Kim et al [4] found that lamb loins aged for 3 wk prior to freezing showed more accelerated lipid oxidation during display compared to frozen/thawed lamb loins (non-aged).
On the other hand, Ismail et al [28] reported that ground meat made with beef aged for 2 and 3 wk had greater lipid oxidative stability during chilled storage than that prepared with beef aged for 1 wk. Moreover, Farouk and Wieliczko [8] reported that lipid oxidation of beef muscles increased up to 3 wk of post-mortem storage then slightly reduced on the 4th wk, which was likely the formation of malondialdehyde-free amino acids/muscle proteins complexes. Moreover, Arihara and Ohata [29] indicated that the bioactive peptides, with antioxidant activity, could be generated from muscle proteins by endogenous proteolysis during post-mortem ageing. Considering the various factors affecting lipid oxidation of processed meat products, such as formulation, processing, and storage conditions, further studies to determine how aged raw meat materials would affect oxidation stabilities (in particular, pro/antioxidant factors) of processed meat products would be warranted.
CONCLUSION
Ageing lamb loins for at least 3 wk prior to freezing improved protein functionality of meat batter, which led to the decreased cooking loss of model sausages made from the aged meat. In addition, model sausages manufactured with the aged and frozen/thawed loins also had higher lipid oxidative stability compared to their counterparts from non-aged lamb loins prior to freezing. Therefore, post-mortem ageing of raw meat prior to freezing could be an effective strategy to improve further WHC and lipid oxidative stability of processed/restructured meat products. Further studies evaluating the effects of aged/frozen/thawed meat on sensory and microbial properties of the final products should be warranted to determine the practical efficacy in the utilization of aged/frozen/thawed meat for manufacturing processed meat products.
ACKNOWLEDGMENTS
This project was supported by the AgResearch Core Fund (67231). The authors would like to acknowledge Kevin Taukiri, Robert Kemp and Carolijin van der Stok for sample and data collections and Dr. Maryann Pirie for the statistical analysis.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.