Silage preparation and fermentation quality of natural grasses treated with lactic acid bacteria and cellulase in meadow steppe and typical steppe
Article information
Abstract
Objective
In order to improve fermentation quality of natural grasses, their silage preparation and fermentation quality in meadow steppe (MS) and typical steppe (TS) were studied.
Methods
The small-scale silages and round bale silages of mixed natural grasses in both steppes were prepared using the commercial lactic acid bacteria (LAB) inoculants Chikuso-1 (CH, Lactobacillus plantarum) and cellulase enzyme (AC, Acremonium cellulase) as additives.
Results
MS and TS contained 33 and 9 species of natural grasses, respectively. Stipa baicalensis in MS and Stipa grandi in TS were the dominant grasses with the highest dry matter (DM) yield. The crude protein (CP), neutral detergent fiber and water-soluble carbohydrate of the mixed natural grasses in both steppes were 8.02% to 9.03%, 66.75% to 69.47%, and 2.02% to 2.20% on a DM basis, respectively. All silages treated with LAB and cellulase were well preserved with lower pH, butyric acid and ammonia-N content, and higher lactic acid and CP content than those of control in four kinds of silages. Compared with CH- or AC-treated silages, the CH+ AC-treated silages had higher lactic acid content.
Conclusion
The results confirmed that combination with LAB and cellulase may result in beneficial effects by improving the natural grass silage fermentation in both grasslands.
INTRODUCTION
Meadow steppe (MS) and typical steppe (TS) are important natural steppes that are widely distributed in temperate semi-arid continental climate region and the northern hemisphere boreal and temperate. In China, they are distributed in the northeast, Inner Mongolia, Xinjiang, Qinghai and Tibet Plateau. These steppes play an important role in animal production. However, both steppes are limited in their hay production due to the cold, dry climate, regardless of water use [1]. They occupy about 400.02 million km2 but can only support 13.62 million tons of hay, which provides about only 42% of the animal feed needed in the winter and spring. Local farmers usually begin storing grass in mid-August. During harvest and storage, dry matter (DM) and crude protein (CP) will be lost [2]. Previously, interest has shifted toward natural grass silage as a main feed source for ruminant animals. Not only can it be prepared ahead of time, in late July to early August, which preserves nutritional value, but it can also extend the retention time, facilitating fodder provision throughout the year, regardless of the weather [3].
It is usually difficult to prepare a good silage fermentation from natural grasses because of their lower moisture, water-soluble carbohydrate (WSC) content and lactic acid bacteria (LAB) counts, as well as their higher lactate buffering capacity [4]. Some studies have tried to solve the problem of poor fermentation by using silage additives, such as LAB and cellulase [5,6], which are widely used for silage preparation. The cellulase can enhance fiber degradation and increase WSC content as a substrate for LAB [7], which can convert WSC into lactic acid [8,9]. As a result, the silage pH is reduced and the forage well preserved. However, limited information is available on the preparation and fermentation of natural grass silage treated with microbiological additives in the both grasslands. The present study examined the grassland population, DM yield, fermentation quality, and chemical composition of natural grasses in MS and TS environments. To improve fermentation quality, small-scale silages and round bale silages of mixed natural grasses in both steppes were prepared using LAB inoculant and cellulase enzyme.
MATERIALS AND METHODS
Grassland population and yield analysis
Natural grasses were harvested at full-bloom stage from Hulunbuir MS (48.27°N, 119.44°E), and Xilingol TS (43.46°N, 115.13°E), Inner Mongolia, China on 24 July 2014. Grasses were harvested in three clipping grasslands with sample lines at 500 m length within the fenced exclosure. According to the specific locations, total 10 sample plots in every 50 m were set with signed global positioning system data on each sample line, we took the quadrat with 1 m×1 m to determine the grass species, and three replicated gradients were used to eliminated the random error. Species density was calculated by dividing the number of individuals in the quadrat [10]. Species cover was determined as the proportion (0% to 1%, 1% to 5%, 5% to 10%, 10% to 20%, 20% to 40%, 40% to 60%, 60% to 100%) of the quadrat covered by its canopy [11]. The biomass production of species was determined by the average weight of all quadrates, and the grasses were oven-dried at 65°C for 48 h to estimate the DM biomass [12].
Silage preparation
The grasses in both steppes were harvested at full-bloom stage. Silages were prepared using small-scale fermentation and round bale system. A commercial LAB inoculant Chikuso-1 (CH, Lactobacillus plantarum, Snow Brand Seed Co., Ltd, Sapporo, Japan) and a commercial cellulase enzyme (AC, acremonium cellulase, Meiji Seika Pharma Co., Ltd, Tokyo, Japan) were used as silage additives. AC is produced from Acremonium cellulolyticus, the main composition are glucanase and pectinase, carboxymethyl-cellulase activity is 7,350 U/g. The LAB were inoculated at 20 mg/kg as 1.00×105 colony forming unit (cfu)/g on a fresh matter (FM) basis. AC was added at 10 mg/kg of FM. Silage treatments were designed as control, CH, AC, and CH+AC. The LAB and cellulase were diluted with deionized water, and the additive solution was sprated using an electronic sprayer (Solo, 417, Hamburg, Germany) for addition of round bale silage. For small-scale silage preparation, a hand-held sprayer (SX-MD16E-2, Shixia Holding Co., Ltd, TaiZhou, China) was used for addition. The same amount of deionized water was sprayed on the control treatment. The small-scale silages were prepared by using polyethylene jars (1L capacity, Changgan Co., Ltd, Huizhou, China). Grasses were cut into 10 mm length by using a chopper machine (130DX, ARS Co., Ltd, Osaka, Japan), and were mixed well with or without LAB and cellulase, maximum 1 kg of grasses were packed into the jars. Round bale silages were made using a Rollant round baler (375 RC, Harsewinkel, Germany). The natural grasses in the field were cut and packed continuously into the baler, and these bales were produced with a maximum weight of 200 kg, and approximately 1.20 m diameter and 1.20 m length. These bales were transported to storage place and four layers of polypropylene films (0.03 mm, the DOW Chemical Company, Hayward, CA, USA) were immediately wrapped by using a round bale wrapper (SW5000, Vermeer Manufacturing Co., Ltd, Pella, IA, USA). These bales and jars were stored in outdoor and indoor at temperature 20°C to 26°C. Three replicates per treatments were opened at 60 days of ensiling, fermentation quality and chemical composition were analyzed.
Chemical analysis
The fermentation products of silage were analyzed by using cold-water extract as described by Cai [13]. Silage (10 g) was blended with 90 mL deionized water and kept in a refrigerator at 4°C for 24 h [14]. The pH was measured with a glass electrode pH meter (STARTER 100/B, OHAUS, Shanghai, China), the ammonia-N content was analyzed by using steam distillation of the filtrates [13], the concentration of organic acid were measured by high performance liquid chromatography methods as described by Cai [13]. The DM content of the samples were oven dried at 65°C for 48 h, CP and organic matter (OM) were analyzed by Horwitz and Latimer [15] method. The content of neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined as described by Van Soest et al [16]. The WSC content was determined as described by Thomas [17].
Statistical analyses
Statistical analyses of chemical composition and silage fermentation were performed by one-way analysis of variance using the general linear model procedure of SAS version 9.1 (SAS Institute Inc, 2003). The differences between means were assessed by Tukey’s multiple comparison tests at a significant level of p<0.05 [18].
RESULTS
Grassland population and yield of natural grasses
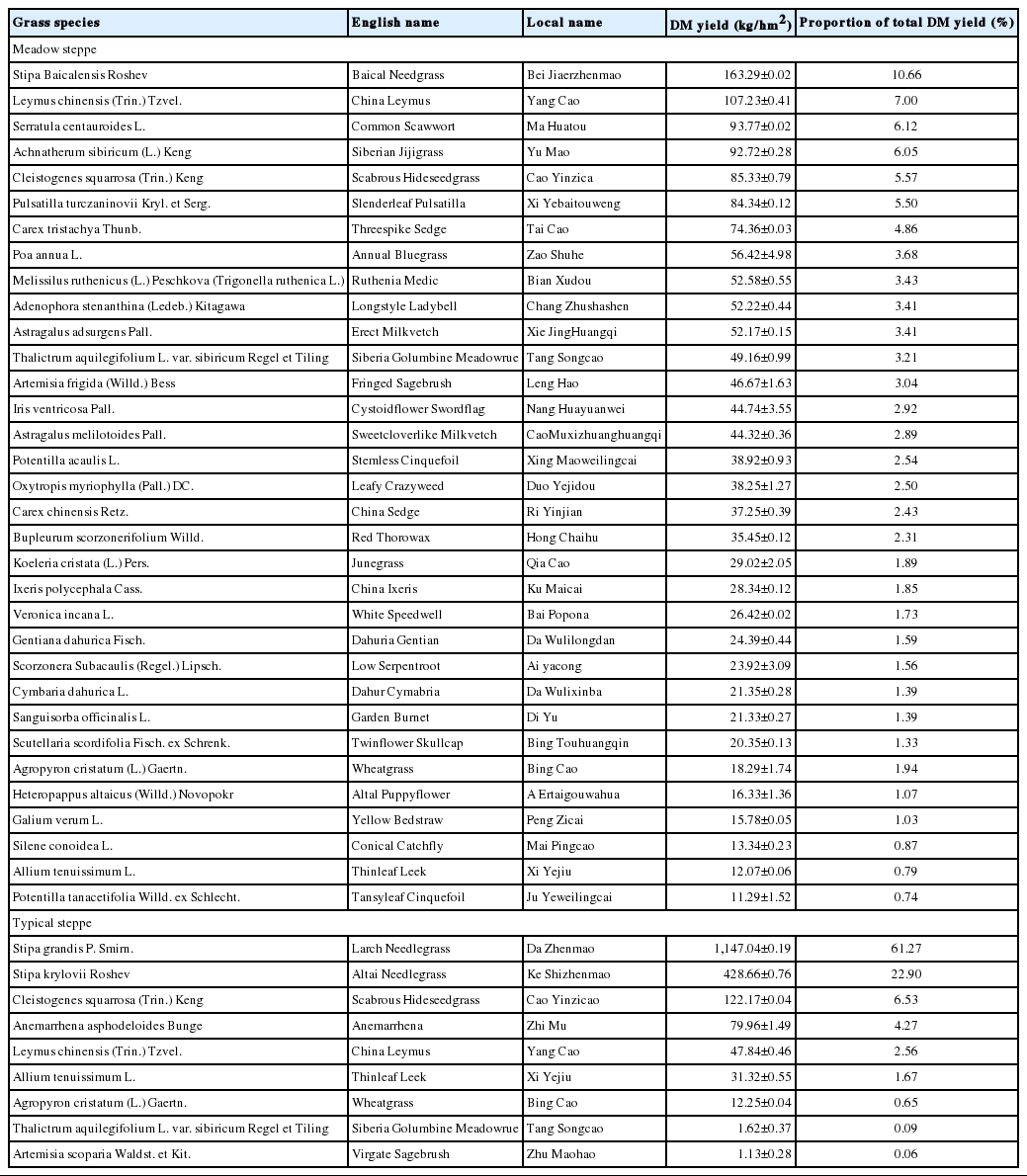
Grassland population and DM yield of MS and TS are shown in Table 1. Based on the DM yield, Stipa Baicalensis, Leymus chinensis, Serratula centauroides, Achnatherum sibiricum, and Cleistogenes squarrosa were the dominant grasses in MS. The DM yield at a high level of order were 163.29 kg/hm2 for Stipa Baicalensis (10.66%, proportion of total DM yields), 107.23 for Leymus chinensis (7.00%), 93.77 for Serratula centauroides (6.12%), 92.72 for Achnatherum sibiricum (6.05%), while other grasses were below 93.00 kg/hm2 in MS. On the other hand, Stipa grandi, Stipa krylovii, Cleistogenes squarrosa, Anemarrhena asphodeloides were the dominant grasses in TS, their DM yield at a high level of order were 1147.04 kg/hm2 for Stipa grandis (61.27%), 428.66 for Stipa krylovii (22.90%), 122.17 for Cleistogenes squarrosa (6.53%), 79.96 for Anemarrhena asphodeloides (4.27%), while other grasses were below 47.84 kg/hm2 in TS. The minimum DM yields were observed from Potentilla tanacetifolia (11.29%) in MS and from Artemisia scoparia (1.13%) in TS, respectively.
Chemical composition of natural grasses
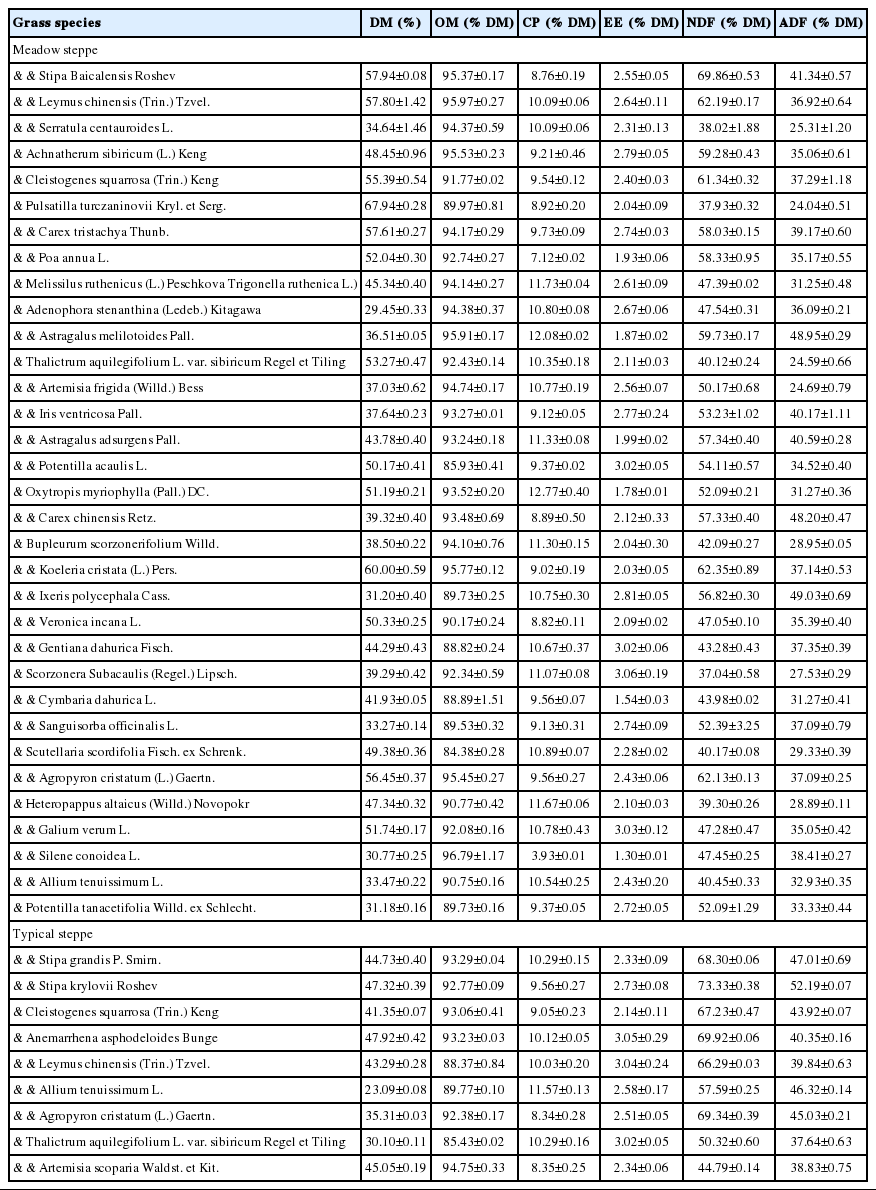
Chemical composition of natural grasses in MS and TS are shown in Table 2. The DM of natural grasses were 29.45% to 67.94% in MS and were 23.09% to 47.92% in TS on a FM basis. In meadow steppe, the highest and the lowest moisture were found in Adenophora stenanthina at 65.30% and Carex tristachya at 34.37% of FM. In TS, the highest and the lowest moisture were found in Thalictrum aquilegifolium at 63.33% and Agropyron cristatum at 47.34% of FM. The OM of both steppes were similar ranging from 84.38% to 96.79% on a DM basis, their ether extract (EE) were 1.30% to 3.06% of DM. The CP of Silene conoidea was the lowest content at 3.93% in MS while other grasses were 7.12% to 12.77% of DM. The NDF and ADF were 37.04% to 69.86% of DM and 24.04% to 48.95% in MS, and were 44.79% to 73.33% and 37.64% to 52.19% of DM in TS, respectively.
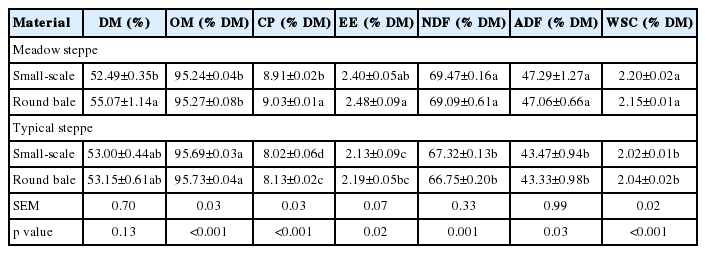
Chemical composition of mixed natural grasses in MS and TS are shown in Table 3. The DM contents of mixed grasses were similar levels ranging from 52.40% to 55.07%, and their OM were also similar with 95% of DM in both steppes. The CP of mixed grasses were 8.91% to 9.03% in MS and were 8.02% to 8.13% in TS. Their NDF and ADF were 69% and 47% of DM in MS, while they were lower more than about 2% and 4% of DM in TS. The WSC contents of mixed grasses in MS (2.15% to 2.20% of DM) were higher (p<0.05) than that in TS (2.02% to 2.04% of DM).
Fermentation quality of mixed grasses silage prepared without or with LAB and cellulase in MS and TS are shown in Table 4. The grasses (G), preparation methods (P), additives (A) and their interaction (G×P, G×A, A×P, and G×A×P) influenced (p<0.001) butyric acid, in addition the additives also influenced (p<0.001) pH, lactic acid, and ammonia-N content. The small-scale and round bale silages in both steppes were showed as similar fermentation results. After 60 days of ensiling, the CH, AC, and CH+AC treatments of small-scale silages and round bale silages in MS and TS were preserved with significantly (p<0.05) lower pH and ammonia-N content, and significantly (p<0.05) higher lactic acid content than those of control. Acetic acid were produced in all silages with 0.38% to 0.57% of FM. Only the control treatment in MS had detected butyric acid (0.16% to 0.25% of FM) and propionic acid (0.11% to 0.17% of FM).
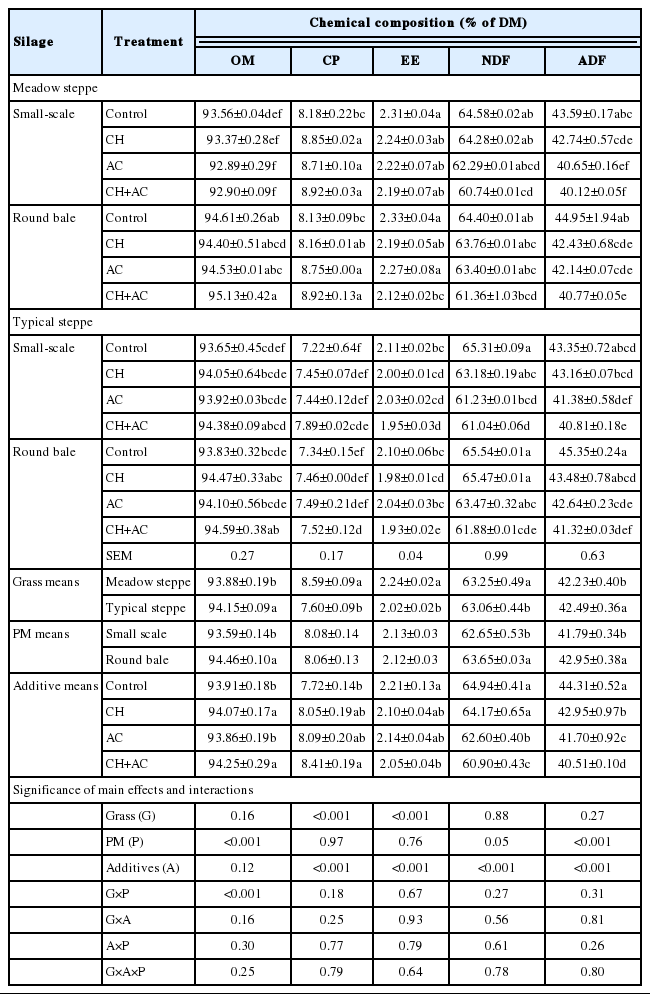
Chemical composition of mixed natural grasses silage in MS and TS were shown in Table 5. The additives influenced (p<0.001) CP, EE, NDF, and ADF. The OM contents of all silages were similar levels raging 92.89% to 95.13% of DM. The CP contents of MS silages (8.13% to 8.92% of DM) were significantly (p<0.05) higher than in the TS silages (7.22% to 7.89% of DM). The CP contents of CH+AC-treated silages were significantly higher (p<0.05) than other treatments, and MS had higher (p<0.05) CP content in the four typies of silages. AC- and CH+AC- treated silages significantly (p<0.05) decreased NDF and ADF contents compared to the control or CH-treatment.
DUSICUSSION
Natural steppes, such as MS and TS, are important feed sources for livestock, and most of the local livestock in China are dependent on these environments. MS occur in the eastern part of the grassland belt, extending westward to the eastern edge of the Inner Mongolian Plateau, China [19,20]. It is reported that the structural species in MS was Sibirian filifolium, and the dominant grasses were Stipa baicalensis and Leymus chinensis. Our study found that the MS contained 33 species of natural grasses, dominated by Stipa baicalensis and Leymus chinensis. However, we did not observe the structural species Sibirian filifolium due to steppe environmental degradation [21].
TS are located west of MS in the Inner Mongolian Plateau. Previous studies have reported the structural species as Stipa grandi and the dominant grasses as Stipa grandi, Stipa krylovii, and Cleistogenes squarrosa [22–24]. We found nine species of natural grasses, dominated by Stipa grandi, Stipa krylovii, Cleistogenes squarrosa, and Anemarrhena asphodeloides, which is consistent with previous studies [25–27]. The structural species had shifted from Compositae to Gramineae, which may lead to easier ensiling. The TS had fewer species than the MS, and the species of Gramineae accounted for a large percentage of total grasses, which could reduce the abundance of other grass species that have an uncertain value for ensiling [28].
Yield is the dominant factor affecting the quality of ensiling [20]. The DM yield of Stipa baicalensis, Leymus chinensis, Serratula centauroides, Achnatherum sibiricum, and Cleistogenes squarrosa accounted for 35.41% of the whole DM yield in MS, whereas Stipa grandi and Stipa kryloviiin accounted for 84.17% of the whole DM yield in TS.
Generally, natural grasses do not grow during the cold season because of the low winter temperatures. Therefore, it is necessary to preserve a feed supply to continuously feed ruminants during the cold season. Silage fermentation is considered the most effective technique for addressing the cold season feed shortage [29].
Silage is now the most common preserved feed for cattle production in many countries, including China [30]. Generally, farm silage is based on natural lactic acid fermentation, in which epiphytic LAB convert WSC into organic acid during the ensiling process. The epiphytic LAB population density has become an important factor in predicting whether to apply LAB in silage [31]. LAB population densities ≥105 (cfu)/g FM usually result in silage that is well preserved [14]. WSC is also an important factor that influences the fermentation quality of silage [32]. A good silage needs a DM >5% WSC for lactic acid fermentation [33]. However, these mixed natural grasses have a relatively low WSC (Table 3). Furthermore, only a few epiphytic LAB are found on these materials [34], suggesting that silage fermentation may need to be improved using LAB inoculants or cellulase enzymes [14].
The LAB- and cellulase-treated silages in both steppes were well preserved, with significantly (p<0.05) lower pH and ammonia nitrogen content and significantly (p<0.05) higher lactic acid content than that of each control. These results are likely explained by the WSC content of the materials and by the numbers and physiological properties of epiphytic LAB. The low WSC content of the mixed grasses could hardly provide enough substrate for LAB fermentation. Added cellulase may degrade the cytoderm and increase the available sugars, thereby providing a substrate for lactic acid fermentation, which is consistent with what Sun et al. found for maize silage [35]. Furthermore, the population of epiphytic LAB is usually very low, and some lactic acid-producing cocci cannot not grow in a pH<4.5. During silage fermentation, the cocci grew rapidly only in the early stage. If the silage pH remained >4.0, then the growth of clostridia was not inhibited, and butyric acid fermentation occurred.
In MS, the control silages were of poor quality, with a high butyric acid content. These results suggest that the inoculant strain Chikuso-1 used in this study is Lactobacilli plnatarum, as it can promote lactic acid fermentation as a homofermentative lactic bacteria and may grow in a low-pH environment [36,37]. Therefore, inoculating silage with these strains may result in beneficial effects by promoting the propagation of LAB and by inhibiting the growth of clostridia, as well as by decreasing ammonia nitrogen, which is an indicator of high-quality fermentation [38,39]. The combination of LAB and cellulase had a greater effect than did treatment with either one alone, showing that these additives promote each other to improve silage fermentation.
The CP content was higher in treatment groups than in controls, and the NDF and ADF contents were lower than those in the controls reported in studies of alfalfa silages [40,41]. The CP content was greatest in the CH+AC- silage, whereas there was no difference between CH- and AC- treatments, which is consistent with previous findings [35]. The lower NDF and ADF contents in the CH+AC- and AC- treatments were probably the result of cellulose-promoted degradation of fiber, which is consistent with the results of Colonbatto [9].
Small-scale fermentation systems were developed and used for LAB screening and silage preparation, because this method can be easy to control under different fermentation conditions [14]. In this study, the silages were prepared using small-scale fermentation and round bale systems. The results showed that small-scale silage values were slightly greater than those observed in round bale silage, because round bale silage with plastic film allows some air permeability [42]. The similarities between the two kinds of silage showed that small-scale fermentation can be used to test the fermentation quality of silage.
These results confirmed that the addition of LAB, cellulase, and their combination benefited silage fermentation by increasing lactic acid, decreasing butyric acid and ammonia nitrogen contents, and improving the silage quality of natural grasses from MS and TS environments.
CONCLUSION
MS and TS contained 33 and 9 species of natural grasses, Stipa Baicalensis and Stipa grandi were the dominant grasses with the highest DM yield in each steppe. Their mixed grasses in both steppes had 8.02% to 9.03% CP and 66.75% to 69.47% NDF of DM. LAB and cellulase, especially their combination could effectively improve fermentation quality of mixed grasses silage in both steppes.
ACKNOWLEDGMENTS
This work was supported by Project of Agriculture Research System (CARS-35), China and the Demonstration of Silage Preparation Technology, Public Interest fund (201303061) for Agro-scientific Research, China. We thank Meiji Seika Pharma Co., Ltd., Tokyo, Japan for providing the commercial cellulase enzyme and Snow Brand Seed Co., Ltd, Sapporo, Japan for providing the commercial LAB inoculant.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.