 |
 |
| Anim Biosci > Volume 30(2); 2017 > Article |
|
Abstract
Objective
Present study explores the effect of hot summer period on the glycolytic rate of early post-mortem meat quality of Ghungroo and Large White Yorkshire (LWY) pig and comparative adaptability to high temperature between above breeds by shifting the expression of stress related genes like mono-carboxylate transporters (MCTs) and heat shock proteins (HSPs).
Methods
Healthy pigs of two different breeds, viz., LYW and Ghungroo (20 from each) were maintained during hot summer period (May to June) with a mean temperature of about 38°C. The pigs were slaughtered and meat samples from the longissimus dorsi (LD) muscles were analyzed for pH, glycogen and lactate content and mRNA expression. Following 24 h of chilling, LD muscle was also taken from the carcasses to evaluate protein solubility and different meat quality measurements.
Results
LWY exhibited significantly (p<0.01) higher plasma cortisol and lactate dehydrogenase concentration than Ghungroo indicating their higher sensitivity to high temperature. LD muscle from LWY pigs revealed lower initial and ultimate pH values and higher drip loss compared to Ghungroo, indicating a faster rate of pH fall. LD muscle of Ghungroo had significantly lower lactate content at 45 min postmortem indicating normal postmortem glycolysis and much slower glycolytic rate at early postmortem. LD muscle of LWY showed rapid postmortem glycolysis, higher drip loss and higher degrees of protein denaturation. Ghungroo exhibited slightly better water holding capacity, lower cooking loss and higher protein solubility. All HSPs (HSP27, HSP70, and HSP90) and MCTs (MCT1, MCT2, and MCT4) in the LD muscle of pigs inclined to increase more in Ghungroo than LWY when exposed to high temperature.
Pigs are quite sensitive to high temperatures and in a tropical country like India this could cause stress and adversely influence the performance of growing pigs and quality of meat. Heat stress reduces growth, alters carcass quality, and compromises efficiency, thus diminishing chances for production of high quality protein for human consumption [1,2]. Pigs synthesize a select group of proteins, called heat shock proteins (HSPs) to cope up with the sudden adverse environmental changes such as heat shock or stress response. The resultant increase and accumulation of the HSPs now gives the stressed cell added protection, thereby allowing for continued cell survival. There is now emerging evidence that HSPs, produced in response to heat stress or other type of cellular stress, may play important role in regulating rate and efficiency of muscle growth and meat quality [3]. There are limited studies available on HSPs in relation to different meat quality parameters. The higher abundance of HSP70 is known to be associated with lower drip loss in pork [4]. Report is also available in abating HSP expression (HSP70 and HSP90) due to transportation stress lowering muscle pH and higher drip loss [5]. Again, HSP90 is significantly associated with initial pH, lightness, yellowness, cooking loss and drip loss which implies that it is recruited in the extreme pH conditions and potentially contribute to the water holding capacity (WHC) and postmortem meat quality [3].
In stressful situations, pigs easily switch their energy metabolism to anaerobic glycolysis and produce lactate. Lactate formed in glycolytic (white) muscle fibers is transported to the liver or to oxidative (red) fibers to be used as a fuel. Moreover, in pigs, the transport of lactate out of muscles is limited by poor capillarization [6] and its utilization as a fuel is limited by the small number of oxidative fibers [7]. So, lactate must be transferred out of the cells to avoid a critical drop in pH. Mono-carboxylate transporters (MCTs) are important membrane proteins facilitating efflux of these lactate and protons from muscles. MCTs transport a monocarboxylate anion and a proton together through the cell membrane according to the electrochemical gradients of substrates [8]. Lactate is quantitatively the most abundant monocarboxylate transported by MCTs, apart from other monocarboxylates, such as butyrate, acetate, propionate and ketone bodies. [9]. MCTs are essential for pH regulation in tissues that rely on glycolysis in their energy metabolism. Their role is also important from meat quality point of view because carcasses that exhibit rapid pH decline after slaughter have been shown to be of poorer meat quality than carcasses with a slow decrease in pH.
This lactate accumulation causes a rapid decline in muscle pH at the early postmortem period [10]. Both the rate and extent of postmortem pH decline significantly influence the protein characteristics, and thus, critically affect ultimate pork quality [11]. Changes in both glycogen and lactate content, which are results of postmortem metabolism, can be very important factors in meat quality. There are no reports of hot summer period on early postmortem glycolysis rate, functional property and meat quality in Ghungroo pig. Therefore, the primary objective of this study was to evaluate the effect of high temperature on the glycolytic rate at early post-mortem, meat quality of Ghungroo breed and Large White Yorkshire (LWY), and to assess their comparative adaptability to summer stress by altering the expression of stress related genes like HSPs and MCTs.
Healthy pigs of two different breeds, viz., LWY and Ghungroo, were selected and 20 male animals from each breed were used in this study. The animals were maintained at Haringhata Pig Farm, Nadia, India following routine farm management practices. The study was conducted during hot summer period (May to June) with a mean temperature about 38°C (Supplementary data). The treatment conditions for the animals were same in both the breeds. To know the effect of summer stress on gene expression and meat quality of our native Ghungroo pig in comparison to LWY, no control animal has been used in this study. The pigs were slaughtered in Haringhata Meat Processing Plant, Nadia, India by electrical stunning during the summer period. The transportation of animals, slaughtering procedures and other methods applied during this study were followed as per the approved guidelines of Institute Ethical Committee and animal welfare committee of Haringhata meat processing plant. At 45 min postmortem, samples were taken from the longissimus dorsi (LD) muscles at the 8th–9th thoracic vertebra [12] for estimation of pH, glycogen, lactate and mRNA extraction study. Following 24 h of chilling, LD muscle was also taken to evaluate pH, glycogen, lactate content, protein solubility and meat quality measurements. For RNA extraction, muscle samples were snap frozen in liquid nitrogen and preserved at −80°C.
Immediately after the onset of bleeding, blood samples were collected from each animal and brought to laboratory to measure the levels of cortisol. Estimation of plasma cortisol was done by Cortisol RIA kit (Immunotech, Beckman Coulter, Prague, Czech Republic). The activity of lactate dehydrogenase (LDH) was assessed using a commercial kit (Span Diagnostic Ltd, Gujrat, India).
For muscle glycogen content, approximately 1.5 g of tissue was minced, suspended in 10 mL of 9% cold perchloric acid (PCA), and thoroughly homogenized for 30 to 45 s with a mechanical tissue disrupter [13]. After centrifugation (15,000 g at 4°C), the supernatants were decanted and saved for glycogen determination. Iodine color reagent, 2.6 mL, prepared by combining 1.3 mL of a solution containing 0.26 g of iodine and 2.6 g of potassium iodide (in 10 mL of distilled water; refreshed daily) with 100 mL of saturated CaCl2, was added to a glycogen standard or to the tissue extracts (0.4 mL). Glycogen standard curves were developed for each set of samples. Linear regression equations were used to determine the glycogen concentrations in the corresponding samples. Glycogen change values were calculated by the difference between glycogen content at 45 min and 24 h postmortem.
Lactate content was determined spectrophotometrically (340 nm) using a commercial kit. Approximately 500 mg of muscle was homogenized for 30 s in 2 mL of 1 M PCA. KOH (2 M) was added to neutralize the solution, and the final volume was made to 10 mL with distilled water. Following 20 min of refrigeration and centrifugation, the lactate concentration was measured [12]. Lactate change values were obtained by the difference between lactate content at 24 h and 45 min postmortem.
Total RNA from tissue was extracted using TRIZOL reagent (Sigma-Aldrich, St. Louis, MO, USA) as per the manufacturer’s instructions. The extracted RNA was dissolved in 30 μL nuclease-free water and the concentration was measured using Nanodrop (Thermo Scientific, Wilmington, NC, USA).
Total mRNA was extracted from muscle using TRI reagent (Sigma, St. Louis, MO, USA) and reverse transcribed into cDNA using an iScriptTM cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Real-time quantitative polymerase chain reaction (RT-PCR) was performed by qPCR in Strategene real time qPCR (Mx3005P, Agilent Technology, Foster City, CA, USA) using SsoFastTM Eva green qPCR kit (Bio-Rad, USA). The primer sets used are shown in Table 1. The thermal profile was an initial 30 s denaturation step at 95°C followed by 40 cycles including denaturation at 95°C for 5 s, gene specific annealing temperature for 10 to 12 s and elongation at 72°C for 10 s followed by a final elongation step at 72°C for 10 min and last cycle at 95°C for 1 min with a gradual increase from 60°C to 65°C for 10 s to 95°C for 15 and 30 s at 0.58°C per sec and continuous fluorescence measurement and a finally cooling down to 40°C. After the end of the run, a cycle threshold (Ct) values and amplification plot for all determined factors were assimilated. Real time PCR efficiencies were determined by amplification of a standardized dilution series and slopes were obtained. The specificity of the desired products was documented using an analysis of the melting temperature, which was product-specific. The amplified PCR products were resolved in 1.5% electrophoresis through agarose gel containing Lab Safe nucleic acid stain (G Biosciences, Santa Clara, CA, USA) and visualized in gel documentation system (GELDOC, Richmond, CA, USA). The relative expression of PCR products was determined [14]. RPS15a was used as housekeeping gene for this experiment.
In order to evaluate the difference in postmortem glycolytic rate of LD muscle; pH, glycogen and lactate were measured at 45 min (pH45 min) and 24 h (pH24 h). Muscle pH was measured at 45 min and 24 h post mortem using a spear type portable pH-meter. Following 24 h of chilling, the LD was taken to evaluate the protein solubility and meat quality traits. Every effort was made to maintain consistency in using the same anatomical location for each procedure. The muscle pH change values were calculated as the difference between the pH measurements at 45 min and 24 h postmortem.
To determine the drip loss, fresh muscle samples that were standardized for surface area and weight were suspended in an inflated bag using small hooks. After a 48 h storage period at 4°C, the muscle samples were weighed again [15]. For cooking loss determination, the samples were placed in vacuum closed plastic bags. After a 20 min cooking period in a temperature controlled bath maintained at 80°C, the bags were cooled with tap water and the samples were taken from the bag, blotted dry and weighed. The drip and cooking loss were expressed as percentage of the initial sample weight. At 24 h post-mortem LD, muscle was used for WHC according to a modified method of Grau and Hamm described by Sierra [16].
In order to determine the solubility of the sarcoplasmic, myofibrillar and total proteins, two extractions were conducted [10]. Briefly, sarcoplasmic proteins were extracted from 1 g of muscle using 10 mL of ice-cold 0.025 M potassium phosphate buffer (pH 7.2). Total proteins were extracted from 1 g of muscle, using 20 mL of ice-cold 1.1 M potassium iodide in a 0.1 M phosphate buffer (pH 7.2). The samples were minced, homogenized on ice and then left on a shaker at 4°C overnight. The samples were then centrifuged at 1,500 g for 20 min. The extracted proteins were determined by biuret method and expressed as mg/g meat sample. Salt soluble protein concentrations were obtained by the difference between total and sarcoplasmic protein solubility.
Serum cortisol, corticotrophin, creatinine kinase, LDH, triiodothyronine (T3) and thyroxine (T4) have been widely used to assess the level of stress in farm animals as well as during pre-slaughter handling [5,17]. The concentrations of cortisol and LDH in blood plasma are shown in Figure 1. In this study, LWY exhibited significantly (p<0.05) higher concentration of serum cortisol than Ghungroo. The reason behind the low cortisol concentration in Gungroo breed might be due to better adaptability to hot and humid climatic condition in India than the cross breed. Shaw and Tume [18] suggested that, while comparing two treatments in terms of stress, the group with the lowest level of cortisol should be considered as least stressed. Therefore, we can conclude that Ghungroo breed is more adaptive and capable enough to cope up the high temperature over LWY. This study also demonstrates that the activity of plasma LDH increased significantly in LWY than Ghungroo due to summer stress. The increased LDH activity indicates that during summer stress, there is intense conversion of pyruvate to lactate (aerobic to anaerobic glycolysis) in LWY than Ghungroo, explaining higher lactate content in postmortem muscle. As the enzyme LDH plays an important role in glycolysis and induction of plasma LDH activity in the present study may indicate the switch from aerobic to anaerobic glycolysis in transported pigs [5,19].
Results revealed that there were no significant differences for glycogen content at 45 min postmortem among the breeds (Table 2) but the Ghungroo breed had slightly higher glycogen content than LWY (1.37 vs 1.21 mg/g). On the contrary, lactate content at 45 min postmortem was significantly different between the breeds. The muscle from Gunghroo breed had a lower lactate level than the LWY (4.52 vs 6.37 mg/g, p<0.01). All the animals were maintained under same treatment condition before and after the slaughter and sampling done at the same site from each pig. However, Hambrecht et al [20] reported that glycogen content can also vary both between and within muscles. There were no significant differences in both the glycogen and lactate contents among the breeds at 24 h postmortem. Other workers also reported no significant differences in porcine muscle glycogen content 3 h after slaughter [21] and even after completion of rigor mortis [22]. Choe et al [12] also reported that there were no significant differences in glycogen and lactate contents after 24 h postmortem in pigs. Our study also corroborates with the above findings indicating no difference in glycogen and lactate contents between the breeds at 24 h postmortem.
Level of muscle glycogen at the time of slaughter is one of the most important factors contributing pH fall due to production of lactic acid which consequently affect meat quality. Further, the rate and extent of postmortem glycolysis at 45 m and 24 h are indicative of meat quality [11]. There are no data available on early postmortem glycolytic rate especially for Indian Ghungroo breed. To evaluate the early postmortem glycolytic rate; changes in muscle pH, glycogen and lactate content were measured at 45 min and at 24 h postmortem (Table 2). It was found that, there was significant difference in pH value at 45 min between the breeds. LWY under high environmental temperature showed significantly lower pH value at 45 min than Ghungroo (6.21 vs 6.43) indicating faster rate of pH fall in early postmortem. Intensity of pH decrease in LWY, particularly at 45 min postmortem was correlated with significantly higher lactic acid production at 45 min postmortem. Considering the metabolic changes that occur in stressed animals, the initial pH will be lowered when compared with non-stressed animals and the rate of pH drop may increase by two to four times and sometimes reaching values below 6.0 in the following hour [23]. But at 24 h postmortem, there was no significance difference in pH values among breeds. Sellier and Monin [24] reported that muscle pH and the R-value are useful parameters for examining the glycolytic rate in the early postmortem at 45 min period. Ryu and Kim [25] classified the muscle samples based on muscle pH45 min and R values in normal (pH≥5.80, R-value <1.05) and fast glycolytic (pH45 min<5.80, R-value>1.05) groups. In this study, the muscle pH45 min of the Ghungroo breed was significantly higher than that of the LYW breed (6.43 vs 6.21, p<0.05).
There were differences in both the glycogen and lactate change values among the breeds. The Ghungroo breed had the highest values for both glycogen (1.04 vs 0.95) and lactate change (3.04 vs 2.35); however, they were not significantly different. Ruusunen and Puolanne [6] reported that glycogen content declines as time goes by, and lactate content shows an opposite tendency due to the metabolism of glycogen via postmortem glycolysis. The relationship between decreased pH and high levels of glycogen is not, however, linear [26] and pH may stabilize in presence of residual glycogen [27], probably because of the disappearance and inactivation at lower pH values of certain co-factors and enzymes [28]. In the present study, muscle from Ghungroo breed had higher glycogen but had low lactate content indicating much slower glycolytic rate at the early postmortem whereas muscle from LWY at 45min postmortem indicated reverse trend. Thus, muscles with lower glycogen and higher lactate content indicate a much faster glycolytic rate at the early postmortem period than muscles with higher glycogen and lower lactate content [12]. The Ghungroo breed under summer stress showed the slower glycolytic rate at the early postmortem period; whereas, under the same environmental conditions, the LWY breed showed the opposite tendency.
The meat quality characteristics for the LD muscle of the both the breeds are presented in Table 3. There were no significant difference in WHC and cooking loss between Ghungroo and LWY. But Ghungroo breed showed slightly better WHC and lower cooking loss than LWY. This could be due to high ultimate pH of meat from Ghungroo. Meat with high pH value has better WHC than meat with low ultimate pH [11]. Incidence of high drip loss represents an economic problem for the swine industry because drip loss leads to significant weight loss in raw, cooked and processed meat [29]. In this study, Gunghroo breed showed significantly lower drip loss than LWY. Higher HSP70 expression and lower drip loss in Ghungroo pig is in agreement with Luca et al [4], who reported a higher abundance of HSP70 which is known to be associated with lower drip loss in pork muscles.
Higher drip loss in case of LWY could be due to high sensitivity to summer stress, high lactate content and faster rate of pH decline during postmortem phase. High drip loss is thought to be influenced by a combination of genetics, variability in energy reserves at time of slaughter, rate of post mortem pH decline and stress [30]. Immonen et al. [31] reported that high concentrations of lactate in hot carcass due to pre-slaughter stress cause a sharp drop in pH and induce protein denaturation, resulting in loss of the water-retention thus causing higher drip loss. Nollet and Toldrá [32] noted that drip is the fluid that accumulates between fiber bundles due to denaturation of proteins and changes occurred during pre-rigor pH fall. Down-regulation of HSP27 can actually accelerate actin disorganization or degradation [33]. The present study also confirmed that due to summer stress, LWY had significantly lower relative mRNA expression of HSP27 than Ghungroo breed. Therefore, it appears that the loss of myofibrillar-related protein function and the subsequent reduction of myofibril integrity could have increased the drip loss in LWY.
The LWY breed showed the slightly lower level of protein solubility, whereas the Ghungroo showed the higher values for protein solubility, but there were no significant differences between the breeds (Table 3). Muscle pH reflects the rate of postmortem metabolism and influences the degree of protein denaturation [24]. Ryu et al [10] reported that initial metabolite levels are closely related to muscle pH, and fast-glycolyzing pigs exhibited much protein denaturation during the early postmortem period. These results could be explained by glycogen and lactate content where, LWY muscles (fast-glycolyzing) showed significantly higher lactate content at 45 min postmortem and higher drip loss, and extent of protein denaturation than muscles from Ghungroo (normal-glycolyzing). In this study, although glycogen content was low at 45 min postmortem, muscles with low levels of lactate showed higher protein solubility and less lightness and drip loss than muscles with high lactate content.
Heat shock proteins play an important role in protein assembly and disassembly, protein folding and unfolding, and refolding of damaged proteins and in the protection of cells and structures [34]. During stress, these proteins have been shown to prevent inappropriate protein aggregation, mediate the transport of proteins for degradation, help proteins to maintain their conformation and even to assist in their repair [35]. The differences in HSPs expression in Ghungroo and LWY pigs may reflect the variations in animal adaptability to sudden environmental changes and various stresses. In this study, the mRNA expression of HSP27 and HSP90 gene was higher in LD muscle of Ghungroo compared to LWY (Figure 2). The results of the present study are in agreement with the findings of Parkunan et al [36] who also reported significant increase in the relative expression of mRNA of HSP27 in the thigh muscle in Ghungroo compared to LWY during summer. Further, HSP27 has been reported to be triggered by a variety of physiologically stressful stimuli such as elevated temperature, ischemia, and oxidative stress [37]. The decrease in HSP90 expression in LWY than Ghungroo has been implied to be disadvantageous as far as recovery of cell membrane function and repairing denatured proteins is concerned, which subsequently lead to reduced WHC and higher drip loss [4,5]. Zhang et al [3] also reported that muscles with a high initial pH (pHi) group (pH>6.4) possessing the higher WHC and lightness, contained the highest HSP90 level, followed by intermediate (6.0 to 6.4) and low pHi groups (pH<6.0). We found similar results where muscle from Ghungroo pig had higher pHi and HSP90 expression and WHC.
Similarly, the relative expression of HSP70 was significantly higher (p<0.01) in Ghungroo compared to LWY. This finding is in accordance with our previous studies in which LWY was found to have lower HSP70 expression than Ghungroo during summer season [36] and in the goat peripheral blood mono-nuclear cells [38]. The increased expression during the summer season could provoke its transcription to protect cells from the damaging effects of heat stress such as the denaturation of proteins, helps in protein refolding, and thereby prevents aggregation of denatured proteins [38]. Various stresses, such as heat stress, oxidative stress, bacterial infection and calorie restriction have been shown to cause abnormal pH and induce the expression of HSPs in skeletal muscle [3]. However, the response to stress can vary considerably between breeds or lines. The difference in mRNA expression of HSPs is breed-specific and may reflect variation in thermal tolerance and adaptability to different environmental stressors.
Poor oxidative capacity, higher percentage of white glycolytic fiber, sparse capillarization and a small number of mitochondria make pigs particularly susceptible to anaerobic stress, which may cause both welfare and meat quality problems. When pigs experience stress, glycogen is converted to lactate through glycolysis. If not transported out of the muscle, lactate and protons may accumulate in muscles, causing pain in living muscle thus affecting meat quality. Lactic acid and other monocarboxylate compounds such as butyrate, acetate, propionate, etc cannot cross the plasma membrane by free diffusion due to dissociation to its respective anion at physiological pH [8] and therefore, require a specific transport mechanism called MCTs [9]. MCTs are important membrane proteins facilitating efflux of lactate and protons from muscles. In this study, relative expression of MCT1 mRNA was found to be non-significantly increased in Ghungroo compared to LWY (Figure 3). Parkunan et al [36] Found a similar MCT1 mRNA expression during summer in pigs. But Sepponen [39] recorded contrasting findings where MCT1 was almost undetectable or insignificant in pig muscles.
The expression of MCT2 and MCT4 in LWY was found to be significantly lower than Ghungroo pigs. This is in agreement with other studies reporting higher expression of MCT2 as well as MCT4 in thigh muscle of Ghungroo pig during summer season [36]. MCT4 has been suggested to be the main lactate transporter at high lactate concentrations, which are seen, for example, during intense exercise [40]. Interestingly, Sepponen [39] also reported that the amount of MCT4 was significantly greater in the muscles of wild boar than in the same muscles of domestic pigs. It is suggested that at rest, acidification is prevented by MCT2, but because the capacity of MCT2 is saturable due to its low Vmax value [41], MCT4 with its high Vmax [40,42] is required to prevent acidification during stress, when the capacity of MCT2 is exceeded. The variation in relative mRNA expression of different MCT isoforms of LWY and Ghungroo breed indicate the difference in capability of facilitating lactate efflux during stressful situations. Therefore, the increase in MCTs in the LD muscle of summer stressed Ghungroo pigs, as observed in the present study, may be advantageous for maintaining pH regulation and muscle acidification by efficient efflux of mono-carboxylates out of muscle, such as lactate, which could have subsequently led to reduced drip loss and protein denaturation.
Our results indicate that LWY exhibited significantly higher plasma cortisol concentration and LDH activity than Ghungroo indicating higher sensitivity to heat stress. The LD muscle from Ghungroo pigs showed higher initial glycogen and lower lactate content at 45min postmortem, indicating a slower post-mortem glycolysis and rate of pH fall compared to LWY. On the other hand, LD muscle of LWY with minimal change in both glycogen and lactate change and with high lactate content at 45 min postmortem showed rapid postmortem glycolysis, higher drip loss, and higher extents of protein denaturation. All three HSPs (HSP27, HSP70, and HSP90) and MCTs (MCT1, MCT2, and MCT4) by real time PCR in the LD muscle of pigs showed an increasing trend in Ghungroo than LWY after summer stress. The results suggested that effect of summer stress on the variation of HSPs and MCTs may play a crucial role in thermal tolerance and adaptation to different climatic conditions, pH regulation, muscle acidification, drip loss, protein denaturation and also in postmortem meat quality development in pig. The current results imply that a possible mechanism for producing relatively poor meat quality in the LD muscle of LYW than Ghungroo pig after summer stress may be due to the decline of different HSPs and MCTs expression. Higher levels of HSPs and MCTs transcripts indicated that Ghungroo pigs are more adaptive towards thermal stress in comparison to the exotic breed, LWY.
ACKNOWLEDGMENTS
This work was supported by grants from the Department of Biotechnology, Govt of India and the authors are thankful to the Director, Indian Veterinary Research Institute, Izatnagar, India for providing necessary facility for conducting this research work.
Figure 1
Effect of summer stress on plasma cortisol and lactate dehydrogenase (LDH) in pigs. (** p<0.01, Mean±standard error). LWY, Large White Yorkshire.
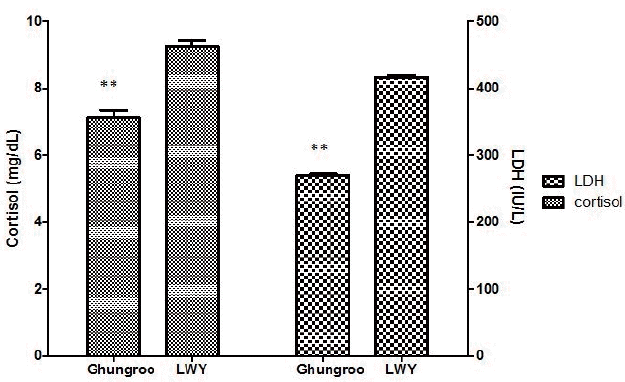
Figure 2
Effect of summer stress on the expression of HSP27, HSP70, and HSP90 in pigs. (* p<0.05,** p<0.01, Mean±standard error, n = 20). HSP, heat shock proteins; LWY, Large White Yorkshire.
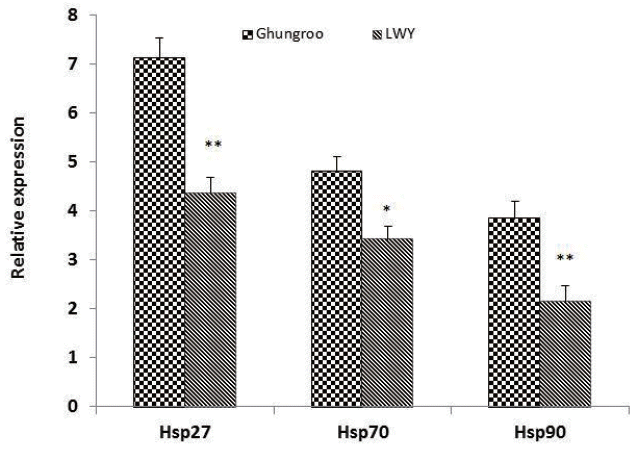
Figure 3
Effect of summer stress on the expression of MCT1, MCT2, and MCT4 in pigs. (* p<0.05, ** p<0.01, Mean±standard error, n = 20). MCT, mono-carboxylate transporters; LWY, Large White Yorkshire.
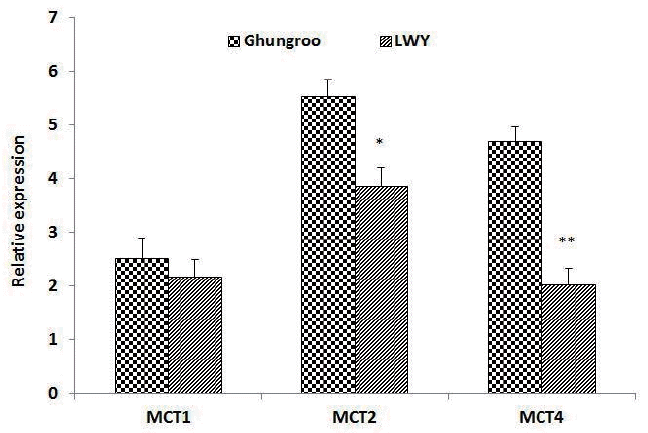
Table 1
List of porcine primers
Table 2
Effect of summer stress on pH, muscle glycogen and lactate content in pig breeds
Table 3
Effect of summer stress on meat quality in pig breeds
REFERENCES
1. Baumgard LH, Rhoads RP, Rhoads ML, et al. Impact of climate change on livestock production. Sejian V, Naqvi SMK, Ezeji T, Lakritz J, Lal R, editorsEnvironmental stress and amelioration in livestock production. 1st edSpringer; 2012. p. 413–68.

2. Berton MP, de Cássia Dourado R, de Lima FBF, et al. Growing-finishing performance and carcass yield of pigs reared in a climate-controlled and uncontrolled environment. Int J Biometeorol 2015; 59:955–60.



3. Zhang M, Wang D, Geng Z, et al. The level of heat shock protein 90 in pig Longissimus dorsi muscle and its relationship with meat pH and quality. Food Chem 2014; 165:337–41.


4. Luca AD, Mullen AM, Elia G, Davey G, Hamill RM. Centrifugal drip is an accessible source for protein indicators of pork ageing and water-holding capacity. Meat Sci 2011; 88:261–70.


5. Yu J, Tang S, Bao E, et al. The effect of transportation on the expression of heat shock proteins and meat quality of M. longissimus dorsi in pigs. Meat Sci 2009; 83:474–8.


6. Ruusunen M, Puolanne E. Histochemical properties of fibre types in muscles of wild and domestic pigs and the effect of growth rate on muscle fibre properties. Meat Sci 2004; 67:533–9.


7. Essén-Gustavsson B. Activity-and inactivity-related muscle adaptation in the animal kingdom. 2nd edChampaigne, IL: Human Kinetic Publishers; 1986.
8. Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol Cell Physiol 1993; 264:C761–C82.

9. Halestrap A, Price N. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 1999; 343:281–99.



10. Ryu Y, Choi Y, Kim B. Variations in metabolite contents and protein denaturation of the Longissimus dorsi muscle in various porcine quality classifications and metabolic rates. Meat Sci 2005; 71:522–9.


11. Scheffler T, Gerrard D. Mechanisms controlling pork quality development: The biochemistry controlling postmortem energy metabolism. Meat Sci 2007; 77:7–16.


12. Choe J, Choi Y, Lee S, et al. The relation between glycogen, lactate content and muscle fiber type composition, and their influence on postmortem glycolytic rate and pork quality. Meat Sci 2008; 80:355–62.


13. Dreiling C, Brown D, Casale L, Kelly L. Muscle glycogen: comparison of iodine binding and enzyme digestion assays and application to meat samples. Meat Sci 1987; 20:167–77.


14. Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 2001; 29:e45



15. Honikel K. How to measure the water-holding capacity of meat? Recommendation of standardized methods. Evaluation and control of meat quality in pigs. Dublin, Ireland: Springer; 1987. p. 129–42.

16. Sierra I. Production of young and heavy lamb in the Aragonese rasa breed. Zaragoza, Spain: Institute of Economy and Productions Livestock of the Ebro; 1973. p. 18
17. Maria G, Villarroel M, Chacon G, Gebresenbet G. Scoring system for evaluating the stress to cattle of commercial loading and unloading. Vet Record 2004; 154:818–21.


18. Shaw F, Tume R. The assessment of pre-slaughter and slaughter treatments of livestock by measurement of plasma constituents—a review of recent work. Meat Sci 1992; 32:311–29.


19. Dzugaj A. Localization and regulation of muscle fructose-1, 6-bisphosphatase, the key enzyme of glyconeogenesis. Adv Enzyme Regul 2006; 46:51–71.


20. Hambrecht E, Eissen J, Newman D, et al. Preslaughter handling effects on pork quality and glycolytic potential in two muscles differing in fiber type composition. J Anim Sci 2005; 83:900–7.


21. Ylä-Ajos MS, Lindahl G, Young JF, et al. Post-mortem activity of the glycogen debranching enzyme and change in the glycogen pools in porcine M. longissimus dorsi from carriers and non-carriers of the RN– gene. Meat Sci 2007; 75:112–9.


22. Aalhus J, Best D, Murray A, Jones S. A comparison of the quality characteristics of pale, soft and exudative beef and pork. J Muscle Foods 1998; 9:267–80.

23. Henckel P, Karlsson A, Oksbjerg N, Petersen JS. Control of post mortem pH decrease in pig muscles: experimental design and testing of animal models. Meat Sci 2000; 55:131–8.


25. Ryu Y, Kim B. Comparison of histochemical characteristics in various pork groups categorized by postmortem metabolic rate and pork quality. J Anim Sci 2006; 84:894–901.


26. Bendall JR. Postmortem changes in muscle. Bourne GH, editorThe structure and function of muscle. New York: Academic Press; 1973. p. 244–309.

27. Przybylski W, Vernin P, Monin G. Relationship between glycolytic potential and ultimate pH in bovine, porcine and ovine muscles. J Muscle Foods 1994; 5:245–55.

28. Sahlin K. Intracellular pH and energy metabolism in skeletal muscle of man with special reference to exercise. Acta physiol Scand Suppl 1978; 455:1–56.

29. van de Wiel DF, Zhang WL. Identification of pork quality parameters by proteomics. Meat Sci 2007; 77:46–54.


30. Fischer K. Drip loss in pork: influencing factors and relation to further meat quality traits. J Anim Breed Genet 2007; 124:12–18.

31. Immonen K, Ruusunen M, Puolanne E. Some effects of residual glycogen concentration on the physical and sensory quality of normal pH beef. Meat Sci 2000; 55:33–8.


32. Nollet LM, Toldrá F. Handbook of muscle foods analysis. 1st edBoka Raton, FL: CRC Press; 2008.
33. Bernard C, Cassar-Malek I, Le Cunff M, et al. New indicators of beef sensory quality revealed by expression of specific genes. J Agric Food Chem 2007; 55:5229–37.


34. Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukocyte Biol 2007; 81:15–27.


35. Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther 1998; 80:183–201.


36. Parkunan T, Banerjee D, Mohanty N, et al. A comparative study on the expression profile of MCTs and HSPs in Ghungroo and Large White Yorkshire breeds of pigs during different seasons. Cell Stress Chaperones 2015; 20:441–9.



37. Rylander MN, Feng Y, Bass J, Diller KR. Thermally induced injury and heat-shock protein expression in cells and tissues. Ann NY Acad Sci 2006; 1066:222–42.

38. Dangi SS, Gupta M, Maurya D, et al. Expression profile of HSP genes during different seasons in goats (Capra hircus). Trop Anim Health Prod 2012; 44:1905–12.


39. Sepponen K. Monocarboxylate transporters and heat shock proteins in domestic pigs in relation to stress and meat quality [dissertation]. Helsinki: University of Helsinki; 2008.
40. Dimmer K, Friedrich B, Lang F, Deitmer J, Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J 2000; 350:219–27.



- TOOLS





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print





