 |
 |
| Anim Biosci > Volume 29(9); 2016 > Article |
|
Abstract
The objective of this research was to estimate genetic parameters and trends for length of productive life (LPL), lifetime number of piglets born alive (LBA), lifetime number of piglets weaned (LPW), lifetime litter birth weight (LBW), and lifetime litter weaning weight (LWW) in a commercial swine farm in Northern Thailand. Data were gathered during a 24-year period from July 1989 to August 2013. A total of 3,109 phenotypic records from 2,271 Landrace (L) and 838 Yorkshire sows (Y) were analyzed. Variance and covariance components, heritabilities and correlations were estimated using an Average Information Restricted Maximum Likelihood (AIREML) procedure. The 5-trait animal model contained the fixed effects of first farrowing year-season, breed group, and age at first farrowing. Random effects were sow and residual. Estimates of heritabilities were medium for all five traits (0.17±0.04 for LPL and LBA to 0.20±0.04 for LPW). Genetic correlations among these traits were high, positive, and favorable (p<0.05), ranging from 0.93±0.02 (LPL-LWW) to 0.99±0.02 (LPL-LPW). Sow genetic trends were non-significant for LPL and all lifetime production traits. Sire genetic trends were negative and significant for LPL (−2.54±0.65 d/yr; p = 0.0007), LBA (−0.12±0.04 piglets/yr; p = 0.0073), LPW (−0.14±0.04 piglets/yr; p = 0.0037), LBW (−0.13±0.06 kg/yr; p = 0.0487), and LWW (−0.69±0.31 kg/yr; p = 0.0365). Dam genetic trends were positive, small and significant for all traits (1.04±0.42 d/yr for LPL, p = 0.0217; 0.16±0.03 piglets/yr for LBA, p<0.0001; 0.12±0.03 piglets/yr for LPW, p = 0.0002; 0.29±0.04 kg/yr for LBW, p<0.0001 and 1.23±0.19 kg/yr for LWW, p<0.0001). Thus, the selection program in this commercial herd managed to improve both LPL and lifetime productive traits in sires and dams. It was ineffective to improve LPL and lifetime productive traits in sows.
Length of productive life (LPL) and lifetime production traits (lifetime number of piglets born alive [LBA], lifetime number of piglets weaned [LPW], lifetime litter birth weight [LBW], and lifetime litter weaning weight [LWW]) are important for commercial swine operations because they affect efficiency of production, costs, and profitability. Sows that have long LPL would be more productive and profitable than sows with short LPL (Stalder et al., 2003; Abell, 2011). Highly productive sows are preferred by commercial swine producers and kept for as long as possible in the production system. Further, sows with longer LPL are likely to be healthier (Tummaruk et al., 2001) and their offspring better defended against infectious microorganisms because they receive higher levels of antibodies from their dams than progeny from sows with shorter LPL (Sobczyńska et al., 2013). Thus, increasing LPL is expected to increase the productivity of sows in the breeding herd as well as the profitability of swine operations. However, swine producers in Thailand have focused their culling and selection on traits measured in individual parities (e.g., litter size at birth and weaning, individual piglet weight and litter weight at birth and at weaning) instead of LPL and lifetime production traits. The target of Thai commercial swine producers has been to produce more and heavier piglets to reduce production costs and maximize profits.
Most Thai swine producers use an open-house system to keep animals throughout the year. Such system exposes sows to more variation under tropical environmental conditions than sows kept in climate-controlled barns. However, Thai swine farms utilize European breeds such as Landrace and Yorkshire originated under temperate climate conditions. Thus, to establish a genetic improvement program for LPL and lifetime production traits in Thailand, genetic parameters for these traits under open-house tropical production conditions are needed. Only a single unpublished study on genetic parameters for LPL, LBA, and LPW exists in Thailand. Keonouchanh (2002) reported low heritability for LPL (0.03 to 0.04), LBA (0.18 to 0.20), and LPW (0.12 to 0.19) and low and positive genetic correlations between LPL and LBA (0.25 to 0.65) in a swine population composed of Duroc, Landrace, and Large White in Northeastern Thailand. Thus, a study involving LPL and lifetime numbers and weights of piglets at birth and at weaning is needed to develop comprehensive swine genetic improvement programs for LPL and lifetime production traits in Thailand. Consequently, the objective of this research were to estimate genetic parameters and trends for LPL, LBA, LPW, LBW, and LWW using data from a commercial swine population composed of purebred Landrace and Yorkshire pigs kept in an open-house system under Thai tropical environmental conditions.
This research utilized a field dataset from a commercial swine farm in Northern Thailand. The original dataset included 3,541 Landrace (L) and Yorkshire (Y) sows. Sow records consisted of sow identification, sow breed, sire breed, dam breed, parity number, sow birth date, farrowing date, number of piglets born alive, number of piglets weaned, weight at birth and weight at weaning for each parity. Parity of sows was classified into 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 and more parities. Only sows that farrowed continuously, had at least one lifetime production trait, and had completed their lifetime production (i.e., known date of first farrowing and date of last weaning) were kept for the study. Sows that were still alive, had missing records, or had their first farrowing at less than 300 or more than 500 d of age were excluded. After the editing process, 3,109 sows (2,271 L and 838 Y) with complete lifetime records in the breeding herd, and had their first farrowing between July 1989 and August 2013 were included in the study.
The LPL was defined as the number of days between age of sow at first farrowing and age of sow at weaning of her last farrowing. The LBA was the number of piglets born alive during the lifetime of a sow. The LPW was the number of piglets weaned over the lifetime of a sow. The LBW was the sum of the birth weights of all piglets born during the lifetime of a sow. The LWW was the sum of the weaning weights of all piglets weaned during the lifetime of a sow.
The swine commercial herd used in this research was located in the province of Chiang Mai, Northern Thailand (18° 47′ 43″ latitude North and 98° 59′ 55″ longitude East; elevation = 310 m above sea level). The average temperature in this area was 27°C (average minimum = 17°C; average maximum = 34.5°C), the average rainfall was 1,218 mm (average minimum = 880 mm; average maximum = 1,457 mm), and the average humidity was 73.2% (average minimum = 37%; average maximum = 99%) over the last thirteen years (Thai Meteorological Department, 2014). Seasons were defined as winter (November to February), summer (March to June), and rainy (July to October). All gilts and sows were kept in an open-house system. Gilts and sows that had their first litter in the same year-season received similar feeding and management. Gilts and non-lactating sows received 2.5 kg/d of feed with 16% crude protein and 3,200 to 3,500 kcal/kg (two feeding times; 07:00 am and 13:00 pm), whereas nursing sows received 5 to 6 kg/d of feed with 17% to 18% crude protein and 4,060 kcal/kg (four feeding times; 07:00 am, 10:00 am, 13:00 pm and 15:00 pm).
Mating was performed by artificial insemination. Estrus was detected by visual appraisal (reddening and swelling of the vulva) and by boar exposure twice a day (morning and afternoon). Replacement gilts were inseminated in their third observed estrus (8 to 9 mo of age and body weight of at least 140 kg). Sows were serviced on the second observed estrus (twice; firstly 12 h after detecting estrus and then 12 h later). Gilts and sows were kept in individual stalls in open-house buildings with dripping, fogging, and fans placed in the farrowing unit approximately 7 d before farrowing. Piglets were weaned when they reached 5 to 7 kg of body weight or 26 to 30 d of age.
Descriptive statistics for LPL, LBA, LPW, LBW, and LWW were obtained with the MEAN procedure of SAS (SAS, 2004). The general linear model procedure of SAS was used to assess the importance of fixed effects on all traits using in single-trait fixed models. The single-trait fixed models for LPL, LBA, LPW, LBW, and LWW contained the effects of first farrowing year-season (73 year-season combinations), breed group (L and Y), and age at first farrowing (10 to 17 mo). Least squares means (LSM) were estimated for all first farrowing year-season and breed group subclasses. Comparison between LSM was done using Bonferroni t-tests.
A five-trait analysis was carried out to estimate variance and covariance components using an Average Information Restricted Maximum Likelihood (AI-REML) algorithm (ASREML; Gilmour et al., 2000). Estimates of variance components were subsequently used to calculate heritabilities, genetic correlations, and phenotypic correlations between LPL and lifetime production traits (LBA, LPW, LBW, and LWW). The 5-trait mixed animal model contained the fixed effects of first farrowing year-season and breed group (L and Y) as subclass fixed effects, and age at first farrowing as a fixed covariate. Random effects were sow and residual. The pedigree file contained 5,525 animals, 690 sires, and 1,512 dams. The genetic parameters were estimated using the following animal model:
where y is the vector of sow records for LPL, LBA, LPW, LBW, and LWW, b is the vector of contemporary groups (first farrowing year-season subclasses) and a covariate for age at first farrowing (mo), ga is vector of additive group genetic effects (L and Y), aa is the vector of random animal additive genetic effects deviated from their breed group, X is an incidence matrix relating sow records to fixed effects in vector b, Za is an incidence matrix relating sow records to random animal additive genetic effects in vector aa, Qa is an incidence matrix relating elements of vector aa to additive genetic groups in vector ga and e is the vector of residual random effects. The assumptions of the model were:
where Ga = G0⊗A, where G0 is a 5×5 matrix of genetic covariances among LPL, LBA, LPW, LBW, and LWW, A is the numerator relationships matrix, ⊗ represents direct product, and R = R0⊗I, where R0 is a 5×5 matrix of residual covariances among LPL, LBA, LPW, LBW, and LWW, and I is an identity matrix. The estimated variance and covariance components were used to compute genetic parameters for all traits (heritabilities, genetic correlations, and phenotypic correlations).
Additive genetic predictions were computed for all sows, sires, and dams in the population using the 5-trait animal model described above and estimates of variance components values obtained at convergence. The estimated breeding value for each animal was computed as the sum of breed group solution and its predicted additive genetic effect deviated from its breed group. Because breed effects are not estimable but breed differences are estimable, breed effects were estimated as deviations from Landrace for all traits. Weighted EBV means for sows, sires and dams were computed for all traits at each first-farrowing year (FFY; 1989 to 2013), where weights were the number of litters per year for sows, sires, and dams. Weighted yearly means for sow, sire, and dam EBV were plotted against FFY to illustrate changes in mean EBV for these animals during the years of the study. Genetic trends for sow, sire, and dam EBV from 1989 to 2013 were computed as linear regression coefficients of mean sow, sire, and dam EBV on FFY with the REG procedure of SAS (SAS, 2004).
Means, standard deviation, minimum, and maximum values for LPL, LBA, LPW, LBW, and LWW are shown in Table 1. The LPL ranged from 21 to 1,596 d, with an average of 680 d. The average for lifetime production traits were 52 piglets for LBA, 46 piglets for LPW, 82 kg for LBW and 337 kg for LWW. First farrowing year-season, breed group, and age at first farrowing affected all traits (p<0.0373 to p<0.0001; Table 2).
The LSM for FFY-seasons ranged from 281.39±141.74 (2013-rainy) to 1,036.88±99.81 (1993-rainy) d for LPL, 27.40±10.11 (2013-rainy) to 70.34±8.56 (1996-rainy) piglets for LBA, 21.01±8.87 (2013-rainy) to 63.90±7.50 (1996-rainy) piglets for LPW, 43.77±13.44 (2001-summer) to 109.95±5.92 (2009-rainy) kg for LBW, and 159.71±66.92 (2013-rainy) to 469.67±25.01 (2009-rainy) kg for LWW. These ranges clearly show that FFY-seasons had large effects on all traits in this population. Variation in management strategies, quality and quantity of feed, as well as variability in climate conditions during the years of the study may largely account for these wide ranges of LSM values.
Gilts that had their first farrowing at younger ages had significantly longer LPL (−34.54±7.25 d/mo; p<0.0001), higher LBA (−2.20±0.52 piglets/mo; p<0.0001), higher LPW (−1.85±0.46 piglets/mo; p<0.0001), heavier LBW (−2.72±0.82 kg/mo; p = 0.0009) and heavier LWW (−11.82±3.49 kg/mo; p = 0.0007) than gilts that had their first farrowing at older ages. These findings were in agreement with results from other authors (Serenius and Stalder, 2007; Sobczyńska et al., 2013) who reported that gilts that started farrowing at younger ages had improved LPL and lifetime production traits. Segura-Correa et al. (2011) indicated that age at first farrowing could be reduced to 330 d and that gilts in an early farrowing program should receive a higher level of nutrition to ensure that they reach puberty at an optimal body weight. Thus, age at first farrowing could be considered an early indicator for LPL and lifetime production traits that could be used to select sows for higher LPL and lifetime productivity.
Yorkshire sows had longer LPL (739.84±16.10 d vs 675.01±12.06 d; p = 0.0008), higher LBA (53.11±1.14 piglets vs 50.14±0.86 piglets; p = 0.0314), higher LPW (47.57±1.02 piglets vs 43.99±0.76 piglets; p = 0.0035), heavier LBW (80.57±1.81 kg vs 76.02±1.36 kg; p = 0.0373) and heavier LWW (329.42±7.65 kg vs 308.26±5.74 kg; p = 0.0163) than L sows in this commercial swine population (Table 3). In contrast, Keonouchanh (2002) found non-significant differences between L and Y sows for LPL, LBA, and LPW. Values of LSM for LPL for Y and L sows here were higher than mean values reported for these breeds in various populations located in temperate regions (489 to 652 d for Y and 493 to 617 d for L; Yazdi et al., 2000a, b; Serenius et al., 2008; Hoge and Bates, 2011; Sobczyńska et al., 2013). The longer LPL for Y sows in this study indicated that if sows were selected based on LPL and lifetime production trait performance of their relatives, a larger number of Y than L sows would likely be chosen as replacements.
Genetic, environmental, and phenotypic variance components for LPL, LBA, LPW, LBW, and LWW in this commercial population are shown in Table 4. Estimates of animal genetic variances for these traits were higher than values reported for Northeastern Thailand (Keonouchanh, 2002), and represented between 17% and 20% of the phenotypic variances estimated for these traits (Table 4). These levels of additive genetic variation indicated that these LPL and lifetime production traits would respond to genetic selection in this commercial swine population.
Estimates of heritabilities and their standard error for all traits are presented in Table 5. The heritabilities were 0.17±0.04 for LPL, 0.17±0.04 for LBA, 0.20±0.04 for LPW, 0.15±0.03 for LBW and 0.19±0.04 for LWW. Heritabilities estimated here were within the range of estimates reported in previous studies (Lόpez-Serrano et al., 2000; Serenius and Stalder, 2004; Serenius et al., 2008; Sobczyńska et al., 2013), and they were higher than those estimated in a swine population composed of Duroc, Landrace, and Large White sows in Northeastern Thailand (Keonouchanh, 2002). The medium size heritabilities obtained here for LPL, LBA, LPW, LBW, and LWW indicate that these traits could be integrated into a selection program to improve lifetime production efficiency in this population. However, because these are traits measured at the end of the productive life of sows, LPL and lifetime production records will only be useful to select future replacement sires and dams. Thus, commercial producers could implement a genetic evaluation and selection strategy that combined information on production traits from early farrowings (e.g., first or first and second) from young animals, and LPL and lifetime production traits from animals that finished their productive life to choose sow and boar replacements.
Estimates of genetic and phenotypic correlations between LPL and lifetime production traits are shown in Table 5. All estimates of genetic and phenotypic correlations among these traits were high and positive (greater than 0.92). These genetic and phenotypic correlation estimates were in agreement with previously reported values for LPL and lifetime production traits (Serenius and Stalder, 2004; Sevón-Aimonen and Uimari, 2013; Sobczyńska et al., 2013), and were substantially higher than results from Northeastern Thailand (Keonouchanh, 2002), where genetic correlations between LPL and lifetime production traits ranged from 0.37 to 0.65 in one farm and −0.14 to 0.25 in a second farm.
Positive correlations here indicated that sows with higher LBA, higher LPW, heavier LBW and heavier LWW tended to have a higher probability to remain longer in the breeding herd. Selecting young boars and gilts for high EBV values of LPL and lifetime production traits will in turn positively influence the profitability of swine operations by increasing revenues from higher EBV sows and reducing boar and gilt replacement costs. Cost reduction would be achieved by preselecting gilts and young boars early in life using LPL and lifetime production records from relatives, thus reducing the number of candidates needed for replacement and the replacement costs. Sows that have higher production efficiency and longer LPL are likely to be more fertile, have more piglets alive at birth and heavier litters at weaning over their lifetime, thus increasing the profitability of the business (Sasaki and Koketsu, 2008). In addition, sows that have higher productivity and remain longer in the breeding herd will also likely be healthier than sows that have shorter herd life (Tummaruk et al., 2001).
The high genetic correlations among LPL and lifetime production traits obtained here ensure that selection for lifetime production traits will result in indirect improvement of LPL because sires and dams with higher EBV for lifetime production traits will also tend to have higher progeny means for LPL. As indicated above, computing preliminary EBV for gilts and young boars using records from relatives would be a good tool to choose a smaller group of superior young animals before sending them to the breeding unit. This strategy would help keep a consistent intensity of selection on these traits, stabilize genetic trends, and reduce replacement costs.
Mean yearly EBV for sows, sires, and dams for LPL, LBA and LBW between 1989 and 2013 are shown in Figure 1 to 3. Figures of genetic trends for LPW and LWW (data not shown) were similar to those for lifetime litter traits at birth. Dam genetic trends were positive and significant for all traits (p<0.0001 to p<0.0217), whereas sire genetic trends were negative for LPL and all lifetime production traits (p = 0.0007 to p = 0.0487; Table 6). Thus, it appears that sows were more consistently chosen based on number of piglets born alive and litter weight at birth or at weaning, whereas sires may have been chosen for other traits such as growth. Sow genetic trends were small and non-significant for all traits resulting from the positive genetic trends for their dams and the negative genetic trends for their sires (Table 6). The EBV yearly means for all traits tended to decrease between 1989 and 2001 for sows, sires, and dams. After 2001 the pattern of EBV yearly means differed in sows, sires, and dams. Sire EBV yearly means decreased from 2001 to 2006 then they decreased until 2013. Conversely, dam EBV yearly means continued to increase for LPL and life production traits until 2012 then they dropped in 2013. Sow EBV yearly means also increased from 2001 to 2012, then they dropped in 2013 to their previous levels in 2011, showing values intermediate between those of dams and sires. The smaller differences between sire and dam mean EBV for lifetime production traits from 1989 to 2001 indicated that sires may have been chosen based on phenotypic records for production traits (i.e., number of piglets born alive and litter weight at birth or at weaning) of their dams and perhaps some close relatives (e.g., sisters, aunts) during those years. Conversely, the larger differences between sire and dam EBV from 2002 to 2013 indicated that sires may have been chosen for traits other than number of piglets born alive and litter weight at birth or at weaning. Although precise information on the boar selection strategy in this population was unavailable, if young boars were preferentially chosen based on own growth performance during those years, bigger boars from smaller litters (and lower EBV for LPL and lifetime production traits) may have been chosen in larger numbers than smaller boars from larger litters (and higher EBV for LPL and lifetime production traits). Thus, if the primary selection goal in this commercial population were to improve LPL and lifetime production traits, then the sire selection strategy would need to incorporate lifetime production trait information and be consistent across years to avoid sudden drops in yearly mean EBV. As suggested above, a selection program that includes preselection of young boars based on LPL, lifetime production records of close relatives as well as production records from younger female relatives could be implemented. Such multiple-trait evaluation and selection program would help steadily increase the EBV yearly means for sows, sires, and dams in this commercial population.
The medium heritabilities for LPL, LBA, LPW, LBW, and LWW indicated that genetic improvement for all these traits would be feasible in this herd. The high and positive genetic correlations between LPL and lifetime production traits indicated that preliminary EBV for gilts and boars using records from relatives could be used to preselect young animals to improve LPL, LBA, LPW, LBW, and LWW. Improvement of LPL and lifetime production traits would be expected to lower gilt and boar replacement costs as well as increase production efficiency and profitability of this swine operation.
ACKNOWLEDGMENTS
The authors are thankful for the financial support from the Royal Golden Jubilee project (PHD/0230/2553) of the Thailand Research Fund (TRF), the University of Florida for supporting the training of the first author as a research scholar, and the Four T Co., Ltd for providing the phenotypic information for this research.
Figure 1
Genetic yearly means of sow, sire, and dam estimated breeding values for length of productive life (LPL) from 1989 to 2013.
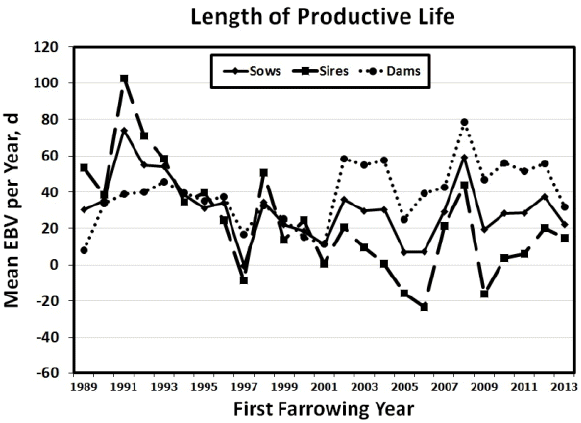
Figure 2
Genetic yearly means of sow, sire, and dam estimated breeding values for lifetime number of piglets born alive (LBA) from 1989 to 2013.
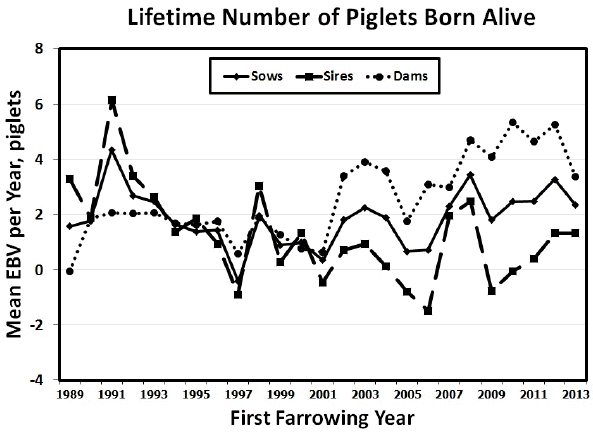
Figure 3
Genetic yearly means of sow, sire, and dam estimated breeding values for lifetime litter birth weight (LBW) from 1989 to 2013.

Table 1
Descriptive statistics for length of productive life and lifetime production traits
Table 2
Levels of significance for factors included in the single-trait fixed model
Table 3
Least squares means and SE per breed group for length of productive life and lifetime production traits
| Traits | Breed group | |
|---|---|---|
|
|
||
| Landrace | Yorkshire | |
| Length of productive life (d) | 675.02±12.06b | 739.84±16.10a |
| Lifetime number of piglets born alive (piglets) | 50.14±0.86b | 53.11±1.14a |
| Lifetime number of piglets weaned (piglets) | 43.99±0.76b | 47.57±1.02a |
| Lifetime litter birth weight (kg) | 76.02±1.36b | 80.57±1.81a |
| Lifetime litter weaning weight (kg) | 308.26±5.74b | 329.42±7.65a |
Table 4
Estimates of variance components for length of productive life and lifetime production traits
| Traits | Variance components1 | ||
|---|---|---|---|
|
|
|||
|
|
|
|
|
| LPL (d2) | 27,889.70±6,267.35 | 138,945.00±6,158.91 | 166,800.00±4,471.00 |
| LBA (piglets2) | 138.09±30.69 | 697.43±30.39 | 835.50±22.33 |
| LPW (piglets2) | 136.69±26.54 | 530.75±24.79 | 667.40±18.11 |
| LBW (kg2) | 323.35±73.82 | 1,771.88±75.02 | 2,095.00±55.78 |
| LWW (kg2) | 7,333.31±1,432.29 | 30,308.40±1,368.94 | 37,640.00±1,016.00 |
Table 5
Heritability (±SE; diagonal), phenotypic (±SE; below diagonal), and genetic correlation (±SE; above diagonal) estimates between length of productive life and lifetime production traits
Table 6
Genetic trends for LPL and lifetime production traits for sows, sires, and dams
REFERENCES
Abell CE. 2011. Evaluation of Litters per Sow per Year as a Means to Reduce Non-productive Sow Days in Commercial Swine Breeding Herds and Its Association with other Economically Important Traits. MS Thesis. Iowa State University; Ames, IA, USA:

Gilmour AR, Cullis BR, Welham SJ, Thompson R. 1999. NSW Agriculture Biometric Bulletin No. 3. ASREML Reference Manual. NSW Agriculture; Orange, NSW, Australia:
Hoge MD, Bates RO. 2011. Developmental factors that influence sow longevity. J Anim Sci 89:1238–1245.


Keonouchanh S. 2002. Genetic Analysis of Sows Longevity and Lifetime Productivity in Two Purebred Swine Herds. MS Thesis. Chulalongkorn University; Bangkok, Thailand:
López-Serrano M, Reinsch N, Looft H, Kalm E. 2000. Genetic correlations of growth, backfat thickness and exterior with stayability in large white and landrace sows. Livest Prod Sci 64:121–131.

SAS. 2004. SAS Online Doc, Version 9.0. SAS Institute Inc; Cary, NC, USA:
Sasaki Y, Koketsu Y. 2008. Sows having high lifetime efficiency and high longevity associated with herd productivity in commercial herds. Livest Sci 118:140–146.

Segura-Correa JC, Ek-Mex EJ, Alzina-López A, Magaña-Monforte JG, Sarmiento-Frandco L, Santos-Ricalde RH. 2011. Length of productive life of sows in four pig farms in the tropics of Mexico. Trop Anim Health Prod 43:1191–1194.


Serenius T, Stalder KJ. 2004. Genetics of length of productive life and lifetime prolificacy in the Finnish Landrace and Large White pig populations. J Anim Sci 82:3111–3117.


Serenius T, Stalder KJ. 2007. Length of productive life of crossbred sows is affected by farm management, leg conformation, sow’s own prolificacy, sow’s origin parity and genetics. Animal 1:745–750.


Serenius T, Stalder KJ, Fernando RL. 2008. Genetic associations of sow longevity with age at first farrowing, number of piglets weaned, and wean to insemination interval in the Finnish Landrace swine population. J Anim Sci 86:3324–3329.


Sevón-Aimonen ML, Uimari P. 2013. Heritability of sow longevity and lifetime prolificacy in Finnish Yorkshire and Landrace pigs. Agric Food Sci 22:325–330.

Sobczyńska M, Blicharski T, Tyra M. 2013. Relationships between longevity, lifetime productivity, carcass traits and conformation in Polish maternal pig breeds. J Anim Breed Genet 130:361–371.


Stalder KJ, Lacy RC, Cross TL, Conatser GE. 2003. Financial impact of average parity of culled females in a breed-to-wean swine operation using replacement gilt net present value analysis. J Swine Health Prod 11:69–74.
Thai Meteorological Department. 2014. Weather report for 2002 to 2014. Weather Station Number 327501; Chiang Mai, Thailand:
Tummaruk P, Lundeheim N, Einarsson S, Dalin AM. 2001. Reproductive performance of purebred Hampshire sows in Sweden. Livest Prod Sci 68:67–77.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





