 |
 |
| Anim Biosci > Volume 29(6); 2016 > Article |
|
Abstract
As the most common neurodegenerative diseases, Alzheimer’s disease (AD) and Parkinson’s disease (PD) are two of the main health concerns for the elderly population. Recently, microRNAs (miRNAs) have been used as biomarkers of infectious, genetic, and metabolic diseases in humans but they have not been well studied in domestic animals. Here we describe a computational biology study in which human AD- and PD-associated miRNAs (ADM and PDM) were utilized to predict orthologous miRNAs in the following domestic animal species: dog, cow, pig, horse, and chicken. In this study, a total of 121 and 70 published human ADM and PDM were identified, respectively. Thirty-seven miRNAs were co-regulated in AD and PD. We identified a total of 105 unrepeated human ADM and PDM that had at least one 100% identical animal homolog, among which 81 and 54 showed 100% sequence identity with 241 and 161 domestic animal miRNAs, respectively. Over 20% of the total mature horse miRNAs (92) showed perfect matches to AD/PD-associated miRNAs. Pigs, dogs, and cows have similar numbers of AD/PD-associated miRNAs (63, 62, and 59). Chickens had the least number of perfect matches (34). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses suggested that humans and dogs are relatively similar in the functional pathways of the five selected highly conserved miRNAs. Taken together, our study provides the first evidence for better understanding the miRNA-AD/PD associations in domestic animals, and provides guidance to generate domestic animal models of AD/PD to replace the current rodent models.
Neurodegenerative diseases (NDDs) are a family of disorders characterized by progressive loss of neuronal structure and function, resulting in neuronal death (Maciotta et al., 2013). Alzheimer’s disease (AD) is the most common form of dementia in people over 65 years of age. The disease is characterized by progressive neuronal loss and inflammation and affects memory, language, behavior, and cognition (Cummings et al., 1998). The primary pathological features of AD are by amyloid-β (Aβ) deposition, neurofibrillary tangle formation, and extensive neuronal degeneration in the brain. Aβ is derived from the sequential cleavage of amyloid precursor protein (APP) by beta-site APP-cleaving enzyme 1 and the γ-secretase complex (Kragh et al., 2009). Parkinson’s disease (PD), the second most common NDD, is estimated to occur in approximately 1% of individuals >60 years of age, with 4.1 to 4.6 million people affected worldwide (Maciotta et al., 2013). PD is a progressive neurodegenerative disorder characterized clinically by bradykinesia, tremor, rigidity, and eventually postural instability (Shtilbans and Henchcliffe, 2012). These symptoms are attributed to a loss of dopaminergic neurons of the substantia nigra. The pathology spreads to other brain regions, including the amygdala, cingulate gyrus, and higher cortical regions, resulting in the development of dementia and psychosis. The disease itself is quite heterogeneous, and symptom progression is variable (Mouradian, 2012).
MicroRNAs (miRNAs), a class of small (18 to 24 nucleotides), are endogenous and non-coding RNAs which bind to target mRNAs so as to regulate protein expression by repressing translation or by promoting degradation of the target mRNAs (Wang et al., 2013) or by enhancing translation at the post-transcriptional level in the RNA-induced silencing complex (Vasudevan et al., 2007). An increasing number of studies have identified dysregulation of miRNAs in NDDs neurodegenerative disease and suggest that alterations in the miRNA regulatory pathway could contribute to the disease pathogenesis (Villa et al., 2013; Tiribuzi et al., 2014). An estimated 70% of miRNAs are expressed in the brain and many are brain-specific or brain-enriched. The miRNAs act by binding to the 3′ untranslated region of mRNAs, and inhibiting translation, causing down-regulation of specific targets. A single miRNA can bind to multiple mRNAs and can therefore fine-tune the expression and function of numerous proteins. Conversely, several miRNAs can regulate the expression and function of a single protein (Muller et al., 2014). The first study that described abnormal expression of miRNAs in AD was published in 2007 (Lukiw, 2007). Three miRNAs, miR-9, miR-125b, and miR-128, were up-regulated in the hippocampus of patients with AD compared with age-matched control subjects.
Successful studies of human diseases require appropriate animal models. An integrated resource of AD-associated miRNA (ADM) and PD-associated miRNA (PDM) data in animals would provide the veterinary research community with an invaluable resource to identify AD- or PD-related miRNA subsets from their animal model experimental data. While databases for human ADM and PDM are publicly available, there is no integrated resource for ADM and PDM in domestic animals. PubMed articles are the only major reliable source of information for data on disease-associated miRNAs. However, there are very few PubMed articles currently (as of 12/31/2014) documenting ADM or PDM in domestic animals compared to those on humans and mice. The utilization of animal models is crucial for any biomedical research on AD and PD processes at the cellular and molecular levels, and for developing novel therapies. A transgenic animal model may also be used to examine the pathogenic mechanisms of AD/PD. Furthermore, these models may aid in the development of vaccines or improve current treatment strategies. Identification of miRNA-AD/PD associations in domestic animals is critical for understanding the role of miRNAs in the pathophysiology of these diseases and may provide guidance to generate domestic animal models of AD/PD to replace the current rodent models.
The main objective of our study was to identify animal miRNA homologs of published human AD- and PD-associated miRNAs in cow, chicken, pig, horse, and dog genomes using homology-searching techniques. Using the current available resources on human and animal miRNAs, we identified potential ADM and PDM in domestic animals based on integrated computational and manual approaches including assessment of the sequence similarities and functional relationships between miRNAs associated with human diseases and their animal orthologs. These predictions will serve as a resource to facilitate hypothesis-driven research in domestic animals, which upon experimental verification in animals could suggest alternative animal models for human AD or PD and strategies for developing therapeutic measures.
Two datasets, on ADM and PDM, were collected from the PhenomiR database 2.0 (December, 2014), the Human MicroRNA Disease Database (HMDD) and miR2Disease database (December, 2014). Only data tested on human AD/PD were retained. Because the same diseases or miRNAs might have different names in the databases, all of the disease terms were annotated with the most commonly used vocabularies of the Unified Medical Subject Headings (MeSH), and the miRNAs were named according to miRBase Release 21.0 (http://www.mirbase.org/). All of the disease terms were manually classified into different disease classes. After removing duplicate data, the remaining data on ADM and PDM from all three databases were integrated as a baseline for searching animal homologs.
We used the human ADM and PDM (from HMDD, PhenomiR, and miR2Disease databases) to extract corresponding mature miRNA nucleotide sequences from miRBase (http://www.mirbase.org/, Release 21.0). We also downloaded all sequences of mature and precursor (pre) miRNAs for cows, chickens, pigs, horses, and dogs from miRBase Release 21.0 and then used a VetBioBase tool (http://vetbiobase.igbb.msstate.edu/microrna.php) to identify cow, chicken, pig, horse, and dog mature miRNA sequences that were 100% identical to sequences of human ADM and PDM.
We used the database miRFocus (http://mirfocus.org/) to predict potential human miRNA target genes. The website describes miRFocus as a human miRNA information database, and is an open-source web tool developed for rapid analysis of miRNAs. First, putative miRNA target genes were retrieved and merged from 5 target prediction algorithms: MiRanda (http://www.microrna.org), MirTarget2 (miRDB: http://mirdb.org), PicTar (http://pictar.mdc-berlin.de), TargetScan (http://www.targetscan.org) and DIANA microT (http://diana.cslab.ece.ntua.gr/microT); together with four validated miRtarget gene databases: MiRecord (http://mirecords.biolead.org), miR2Disease (http://www.mir2disease.org), TarBase (http://diana.cslab.ece.ntua.gr/tarbase) and miRTarbase (http://mirtarbase.mbc.nctu.edu.tw). To enhance the accuracy and reliability of target prediction, common target genes shared by at least three algorithms were included in the target pool. Next, the target pool of input miRNAs was mapped to DAVID (The Database for Annotation, Visualization and Integrated Discovery) database in order to identify animal orthologs.
To elucidate the enriched biological pathways in detail and further unravel the global function of a large number of genes, either predicted human target genes of five selected miRNAs identified from the study or animal orthologs were submitted to DAVID bioinformatics resources (http://david.abcc.ncifcrf.gov) (Wang et al., 2013; 2015), a freely accessible biological database and integrative analytic tool. First, the gene list was uploaded and identifier type was selected coordinately. For instance, we uploaded a gene list with identifiers as “official gene symbol”. Next, the appropriate species from which the gene list originated was indicated. Eventually, the gene list was condensed into gene functional groups and incorporated into Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathways (http://www.genome.jp/kegg). The significance of each functional group or pathway was evaluated by Fisher’s exact test and represented by p-values. The remaining p values were minus log10-transformed after replacing all the zero values with the lowest non-zero values. A high score for a gene group indicates that members of that group are likely to play important functional roles in a given study. We performed functional enrichment analysis by using KEGG pathways to test their functional coherence and meaningfulness. KEGG pathways mainly include cellular processes related to metabolism and biosynthesis. Pathways with p-value <0.01 as revealed by the hypergeometric test were considered statistically significant in the present study. To visualize the distinctness of the cooperative modules in terms of functional enrichment, we created heat maps of the significances using HemI (Heatmap Illustrator, version 1.0, Huazhong University of Science and Technology, Wuhan, China) tool. For further analysis of the data in heat maps, the hierarchical clustering algorithms were also integrated. To calculate the distance, average linkage clustering (default):
and Pearson distance (default):
were adopted.
In order to gain a full understanding of the constructed networks, the open source network visualization software Cytoscape version 3.0 was applied to define the modules in the miRNA-AD-PD association network and miRNA-animal network. Cytoscape is an open source software project for integrating biomolecular interaction networks with high-throughput expression data and other molecular states into a unified conceptual framework.
After surveying three human disease-related miRNA databases (PhenomiR, HMDD and miR2Disease, Figure 1A) and classification of different diseases, we found that PhenomiR database had the most neurological disease-associated miRNAs (total 162 neurological disease-associated miRNAs, including 109 ADM and 42 PDM), followed by HMDD (total 121 neurological disease-associated miRNAs, including 25 ADM and 31 PDM). miR2Disease database had the least neurological disease-associated miRNAs (total 44 neurological disease-associated miRNAs, including 24 ADM and 3 PDM). There were 11 ADM and 2 PDM commonly detected among three different databases (Figure 1B and 1C). These three databases contained a total of 121 published human ADM and 70 published human PDM, and 37 miRNAs were co-regulated in AD and PD (Figure 1D and Figure 2).
Currently 2,588 mature miRNAs (1,881 precursors) have been discovered in humans and deposited in the publicly available miRNA database miRBase (Release 21, update June 2014, http://www.mirbase.org). Specifically, previous studies have identified only 453, 793, 435, 411, and 994 mature miRNAs (502, 808, 502, 382, and 740 precursor miRNAs) in dogs, cows, horses, pigs, and chickens respectively (Figure 3A), which were fewer than those reported in humans (2,588 mature, 1,881 precursors) and mice (1,915 mature, 1,193 precursors).
We identified a total of 105 unrepeated human ADM and PDM (Supplementary Table S1) which had at least one 100% identical animal homolog, among which 81 ADM (Figure 4B) showed 100% sequence identity with 241 mature miRNAs from domestic animals and 54 PDM (Figure 4A) showed 100% sequence identity with 161 mature miRNAs from domestic animals (Supplementary Table S1). Over 20% of total mature miRNAs (92) from horses showed perfect matches to ADM/PDM. Pigs, dogs, and cows have almost the same number of ADM/PDM. As expected, chickens had the least number of perfect matches (100% matches) (34), most likely a result of the relative evolutionary distance between birds and mammals (Figure 3B and Figure 4).
The miRNA-disease association network displays the miRNA signatures of specific diseases, which is helpful for studying the pathological mechanisms of these diseases. We identified 37 miRNAs commonly associated with AD and PD based on the miRNA-AD-PD association network using the Cytoscape (Figure 1D and Figure 2). In miRNA-AD-PD association network, the common miRNA signatures between AD and PD were displayed. For example, as shown in Figure 2, both AD and PD shared mir-30a-5p. We found that five human miRNAs (miR-128-3p, miR-204-5p, miR-205-5p, miR-29b-3p, and miR-30b-5p) were associated with both AD and PD, and were highly conserved in all domestic animals included in this study (Table 1). Then, we selected these five unique miRNAs for target prediction and functional analysis (gene ontology [GO] and KEGG pathway analysis).
To identify the potential target genes of the five selected miRNAs associated with both AD and PD, which were conserved in all five domestic animals, target prediction was performed using miRFocus software (http://mirfocus.org). A total of 2149 putative targets were identified in humans (data not shown). The 2,149 putative genes targeted by the five selected miRNAs were utilized to predict their animal orthologs using the DAVID (The Database for Annotation, Visualization and Integrated Discovery) tool. Currently, 1,859, 1,822, 1,760, 698, and 335 targets are conserved in cows, dogs, chickens, horses, and pigs, respectively (data not shown). Next, the predicted human target genes or animal orthologs were submitted to DAVID database in order to identify the function of the selected miRNAs.
To better understand miRNA functions, we subjected the putative target genes and animal orthologs to KEGG pathway and GO analysis. KEGG is a database resource for understanding the high-level functions and utilities of biological systems. Its pathway-based analysis could facilitate the understanding of the biological functions of genes. To identify the pathways related to target genes of the five selected highly conserved miRNAs, we used the DAVID bioinformatics resources to create KEGG pathway and GO annotations.
The significant GO Term of potential targets of the five selected highly conserved miRNAs are shown in Supplementary Table S4. These potential miRNA targets belong to many gene families, which play various roles during biological processes (such as regulation of apoptosis) (Supplementary Table S4). The five selected highly conserved miRNAs were shown to have significant (p<0.01) association in 24 pathways in humans (Figure 5). Interestingly, six pathways were highly significant among humans, dogs, and cows (Figure 5): endocytosis, mammalian target of rapamycin (mTOR) signaling pathway, glioma, neurotrophin signaling pathway, focal adhesion, and mitogen-activated protein kinase (MAPK) signaling pathway. Previous studies have identified the association of these pathways with AD/PD (Sutherland et al., 2009; Ramanan and Saykin, 2013; Chatterjee et al., 2014).
To determine whether miRNAs function correlated with animal species, we analyzed KEGG pathway data of the five highly conserved miRNA target genes and animal orthologs by hierarchical clustering (Figure 5). Animal species that clustered together had the same functional pathway in all cases. Interestingly, the human pathways clustered together with that of the dog in the KEGG pathway heat map analysis, indicating that the five highly conserved miRNA target gene pathways of these two species are still relatively similar (Figure 5). The cluster analysis showed that, perhaps dog is the best species for the functional analysis of AD/PD-associated miRNAs, and is the best domestic animal model for AD/PD, followed by cow and pig.
Genetically modified animal models are fundamental for understanding the pathogenesis of human disease and for developing novel therapeutic strategies. Genetically modified mice have been widely used in studies of human diseases but often these models cannot replicate all the symptoms of human diseases (Chang et al., 2013). In some cases, the mouse is simply not suited for the condition to be studied for human diseases. In other cases the mouse is simply too small for researchers to obtain certain measurements. Recently, some domestic animals such as pigs and dogs have been used to model genetic human diseases (Groenen et al., 2012), because they are more similar to humans than mice in anatomy, physiology, neurobiology, life span, and genetics. Common animal models for AD/PD research are transgenic mice that overexpress a mutant form of the human AD/PD precursor protein and/or enzymes implicated in its metabolic processing (Sarasa and Pesini, 2009). However, rodent models are not sufficient to fully elucidate the pathogenesis of AD/PD because of genetic, physiological, and anatomic differences between mice and humans. Domestic animals such as pigs and dogs are more suitable for examining human disorders than mice, as pigs and dogs have evolved physiologically and genetically in close proximity to humans (Head, 2007). In addition, canine models naturally develop an age-related cognitive dysfunction that mimics several aspects of AD/PD (Head, 2007). Numerous studies using canine cohorts examining several behavioral paradigms have revealed subsets of aged canines with learning and memory impairments (Adams et al., 2000). Thus, the canine model has been identified as a unique model for studying human disorders, such as AD/PD.
In this study, we found a total of 109 ADM and 42 PDM among PhenomiR, HMDD and miR2Disease three databases. Thirty-seven miRNAs were co-regulated in AD and PD (Figures 1 and 2). In particular, neuron-specific miRNAs have been demonstrated to control neuronal differentiation, excitability, and function. These ADM/PDM play a role in a wide range of neurodegenerative pathologies as disease-causing genes, biomarkers, or agents in pathogenesis (Tan et al., 2013; Tiribuzi et al., 2014). We used a homology approach to generate a resource that integrates animal miRNAs data with human AD/PD-associated miRNAs. A total of 241 and 161 mature miRNA sequences from domestic animals were identified that showed 100% sequence identity with 81 human ADM and 54 human PDM (Figure 3, Supplementary Table S1), respectively. Dogs have the most conserved miRNAs that matches perfectly to AD/PD-associated miRNAs. Pigs, dogs, and cows have almost the same number of ADM or PDM. As expected, chickens had the least number of perfect matches. As demonstrated by using miRNA-AD-PD or ADM/PDM-animal association network and target gene functional analysis, it is logical that similar miRNAs perform comparable functions across related species, and therefore diseases correlated with miRNAs in one species may be correlated with homologous miRNA expression and disease in related species. The example of pathway and GO analysis of the five highly conserved miRNAs (Table 1) highlights the methods to link AD/PD-associated elements across species and develop hypothesis-driven investigation in animals. Integrating all the data enabled us to identify AD/PD miRNAs that are found in more than one animal species (Figure 4 and Supplementary Table S1), which indicates the likelihood that these animals also might have common diseases. Identifying miRNAs targeting similar genes across species provides clues to functional orthology.
As indicated in this study, one miRNA can be associated with both AD and PD. For example, five miRNAs (miR-128-3p, miR-204-5p, miR-205-5p, miR-29b-3p, and miR-30b-5p), which have been documented in nearly 900 PubMed articles, are associated with both AD and PD phenotypes and have homologs in four animal species including chicken. For example, previous studies have shown that overexpression of miR-128 attenuates neuronal responsiveness, suppresses motor activity, and alleviates motor abnormalities associated with Parkinson’s-like disease and seizures in mice (Tiribuzi et al., 2014). The study of Tan et al. ( 2013) also demonstrates that miR-128 inhibition in monocytes from patients with AD improves Aβ (1–42) degradation. Moreover, a recent report revealed that Sp1 and its regulatory miR-29b were deregulated in patients with AD, possibly leading to aberrant production of downstream target genes involved in the pathogenesis of AD (Villa et al., 2013). In silico analysis has demonstrated a predicted binding site for miR-205 in the 3′ UTR of LRKK2 and in vitro experiments confirmed a direct inhibition of LRKK2 via miR-205. Finally it was also demonstrated that transfection of miR-205 in the neurons expressing a PD-related LRKK2 R1441G mutant prevented the neurite outgrowth defects (Cho et al., 2013). However, not all human miRNA-related AD/PD may be relevant to all animals. Validation of the AD/PD miRNAs in animals will likely leverage the findings in humans and at the same time improve our understanding of their roles in the pathogenesis, diagnosis, and prognosis of various animal diseases.
The predicted target genes for the five selected highly conserved miRNAs were classified according to KEGG functional annotations to identify pathways that were actively regulated by miRNA in AD and PD. In this study we found that 24 human pathways were significantly (p< 0.01) associated with the five selected highly conserved miRNAs. Interestingly, six pathways were highly significant among the human, dog cow species: endocytosis, mTOR signaling pathway, glioma, neurotrophin signaling pathway, focal adhesion, and MAPK signaling pathway. Previous studies have identified the association of these pathways with AD/PD (Sutherland et al., 2009; Ramanan and Saykin, 2013; Chatterjee et al., 2014). Numerous large-scale approaches are being used to study complex neurodegenerative diseases and endophenotypes in human tissue and animal and other model systems. Unlike individual genes and other isolated molecules, which may not be present in all model systems and may have differential sensitivity for detection with various study designs, pathways and networks are well-conserved and can be evaluated for convergence across diverse methodological approaches (Ramanan and Saykin, 2013). The results of GO term analysis from the predicted miRNA target genes showed that, regulation of apoptosis was one of the significantly biological processes for most species. It has been reported that neuronal apoptosis underlies the symptoms of many human neurological disorders, including AD and PD (Mattson, 2000). Integration of findings to identify pathways and networks with consistent relationships to AD/PD is likely to enhance the development of diagnostic biomarkers and strategies for prevention and treatment.
Currently, the known information about miRNA networks, especially related to AD and PD, is notably sparser than networks associated with other diseases. As more experiments are carried out, there will be adequate data for the external validation and literature verification of further case studies. It will then be possible to compare more AD/PD-associated miRNAs in domestic animals using various computational programs (Li et al., 2014). As the experimental datasets become enriched, computational programs used for prediction of AD and PD microRNAs in domestic animals will perform better, resulting in the development of experimental studies.
To determine whether function of miRNAs correlated with animal species, we analyzed KEGG pathway data of the five highly conserved miRNA target genes and animal orthologs by hierarchical clustering. Interestingly, the human pathways clustered together with that of the dog in the KEGG pathway heat map analysis, indicating that the five highly conserved miRNA target gene pathways of these two species are relatively similar. The cluster analysis showed that, perhaps dog is the best species for the functional analysis of AD/PD-associated miRNAs, and is the best domestic animal model for AD/PD, followed by cow and pig (Figure 5). Our results are similar to those reported by Peterson et al. (2009). It is worth noting that a recent study demonstrated that microRNAs are challenging traditional ideas about the animal family tree. Peterson et al. (2009) sketched out a radically different diagram for mammals: one that aligns humans more closely with dogs and cows than with rodents with the use of miRNAs technique to work out evolutionary relationships (Peterson et al., 2009). Aged dogs naturally develop cognitive deficits and accumulate brain pathology that is similar to aging humans providing a useful model for studying the neurobiological mechanisms underlying age-related cognitive dysfunction (Head and Torp, 2002). The aging canine also shows impairments in visuospatial working memory and executive function (Studzinski et al., 2006). Aged beagle brain accumulates Aβ-peptide that is of the same sequence as humans (Johnstone et al., 1991) and is correlated with decline in cognitive function with age. Therefore, dogs are considered the most authentic model for studying human AD/PD diseases, as these animals typically share a common environment with man.
We performed a system-level analysis by studying the association of miRNAs with AD/PD. In this study, a total of 121 and 70 published human ADM and PDM were identified, respectively. In our study, 81 ADM and 54 PDM showed 100% sequence identity with 241 and 161 miRNAs from domestic animal species, respectively. KEGG pathway analyses suggested that humans and dogs are relatively similar in the functional pathways of the five selected highly conserved miRNAs. Here, we have shown that some AD/PD-associated miRNAs are well conserved across domestic animal species. Moreover, human genes targeted by ADM/PDM are highly conserved in animals. Conservation of both miRNAs and their target genes across human and domestic animal species suggests the likelihood of functional orthological relationship, which may also lead to similar AD/PD in different species. Findings from this study will contribute towards building an advanced animal ADM/PDM resource, identifying miRNA-related AD/PD in animals, and utilizing miRNA disease biomarkers in animal and veterinary research. In the long-term, validating these human ADM/PDM in domestic animals could help develop new large animal models of AD/PD to replace the current rodent models and to identify biomarkers to expedite development of therapeutic measures for human and animal AD/PD.
ACKNOWLEDGMENTS
This study was supported by a grant from the BioGreen 21 Program (No. PJ011126 to NK), Rural Development Administration (RDA) and research grant of Chubgbuk National University in 2014, Republic of Korea.
Figure 1
Statistics of human AD/PD-associated miRNAs. (A) Number of ADM/PDM in human disease miRNA databases as of 12/31/2014. Venn diagram displaying the overlaps of PDM (A) or ADM (C) among HMDD, PhenomiR and miR2Disease three databases. (D) Venn diagram displaying the overlap between ADM and PDM. AD, Alzheimer’s disease; PD, Parkinson’s disease; miRNAs, microRNAs; HMDD, Human MicroRNA Disease Database; ADM, AD-associated miRNAs; PDM, PD-associated miRNAs.
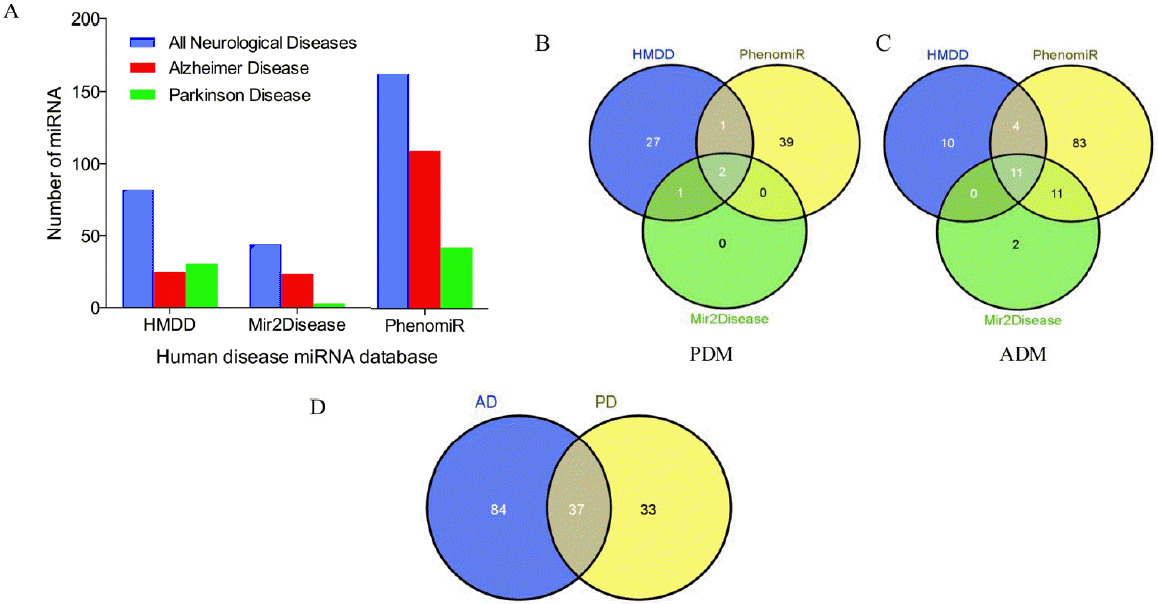
Figure 2
miRNA-AD-PD association network. Alzheimer’s disease (AD) and Parkinson’s disease (PD) are shown as magenta hexagons. The network contains 84 ADM, 33 PDM, 2 diseases (AD/PD), and 37 miRNAs commonly associated with both AD and PD. This network was constructed in Cytoscape interface (Shannon et al., 2003). miRNAs, microRNAs; ADM, AD-associated miRNAs; PDM, PD-associated miRNAs.
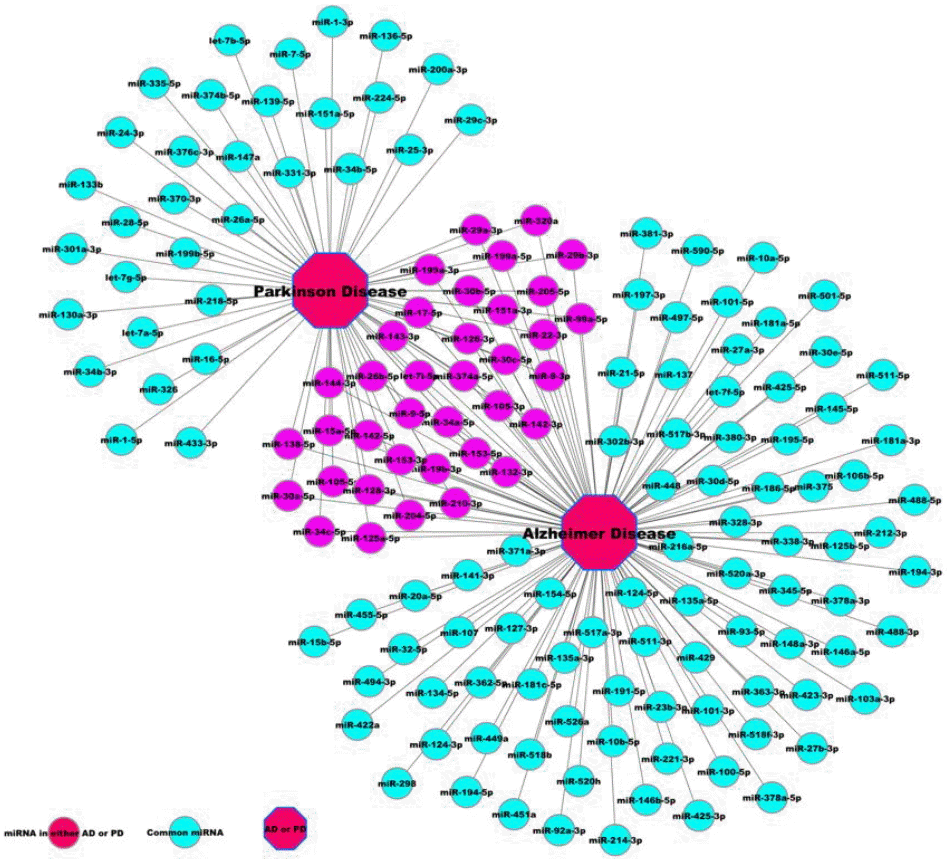
Figure 3
Mature ADM/PDM with counterparts in key domestic animals. (A) Mature and precursor miRNAs of key domestic animals in miRBase Release 21.0. (B) Mature ADM/PDM with counterparts in key domestic animals. Blue bars indicate independent mature ADM with counterparts in the selected animal, red bars indicate common miRNAs between ADM and PDM with counterparts in the selected animal, and green indicates independent mature PDM with counterparts in the selected animal. AD, Alzheimer’s disease; PD, Parkinson’s disease; miRNAs, microRNAs; ADM, AD-associated miRNAs; PDM, PD-associated miRNAs.
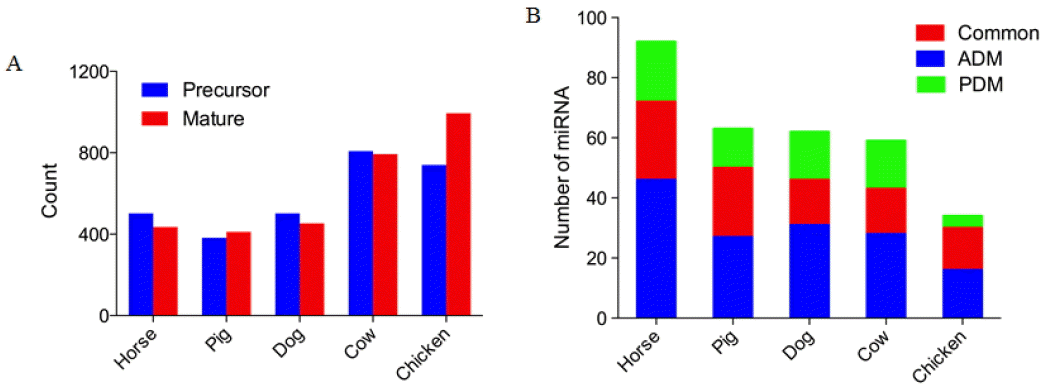
Figure 4
miRNA network of animal counterparts. (A) PDM network of animal counterparts. Domestic animals are shown as magenta hexagons. The network contains 54 PDM that showed 100% sequence identity with the mature miRNAs from five domestic animal species. (B) ADM network of animal counterparts. Domestic animal species are shown as magenta hexagons. The network contains 81 PDM that showed 100% sequence identity with the mature miRNAs form five domestic animal species. This network was constructed in Cytoscape interface (Shannon et al., 2003). AD, Alzheimer’s disease; PD, Parkinson’s disease; miRNAs, microRNAs; ADM, AD-associated miRNAs; PDM, PD-associated miRNAs.
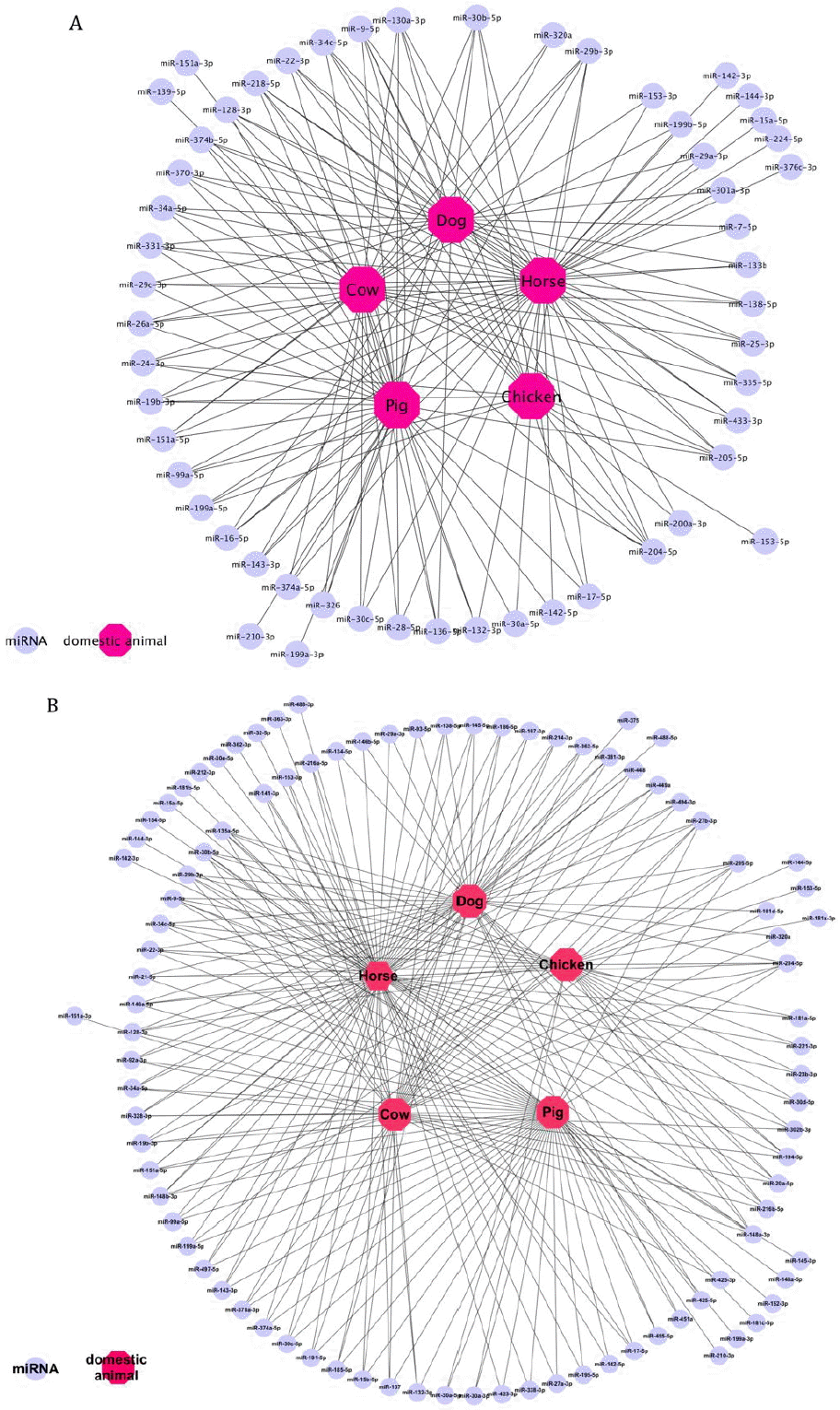
Figure 5
Heatmap of over-represented biological pathways of target genes of the five selected highly conserved miRNAs (miR-128-3p, miR-204-5p, miR-205-5p, miR-29b-3p, and miR-30b-5p). Color gradient represents statistical significance as the −log10 (p-value) in the hypergeometric test for enrichment analysis using Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway. Red indicates significant associations while blue indicates insignificant associations. miRNAs, microRNAs.
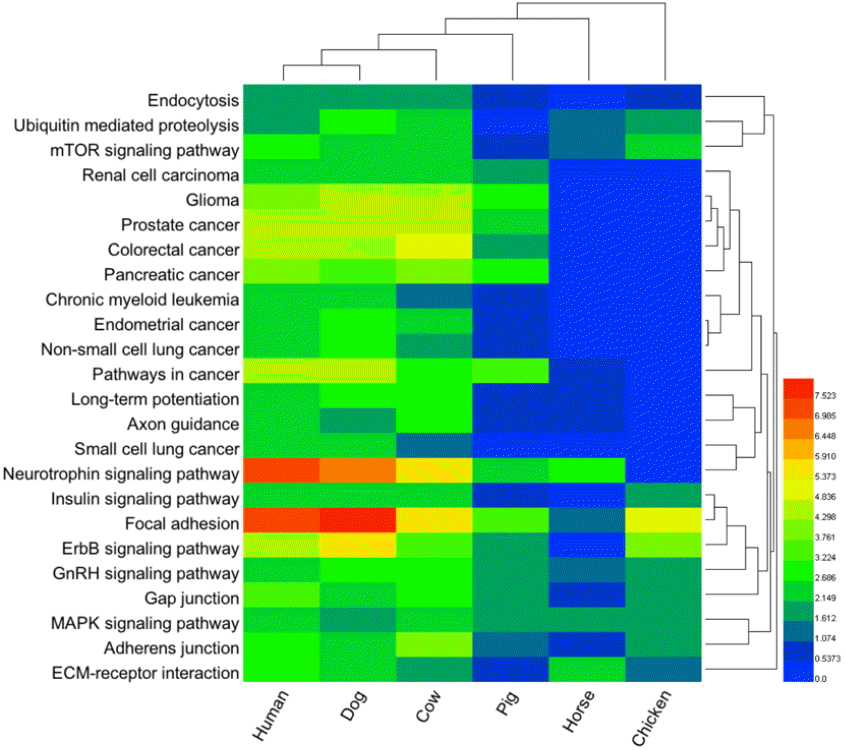
Table 1
Five selected highly conserved miRNAs
REFERENCES
Adams B, Chan A, Callahan H, Siwak C, Tapp D, Ikeda-Douglas C, Atkinson P, Head E, Cotman CW, Milgram NW. 2000. Use of a delayed non-matching to position task to model age-dependent cognitive decline in the dog. Behav Brain Res 108:47–56.


Chang SH, Jung IS, Han GY, Kim NH, Kim HJ, Kim CW. 2013. Proteomic profiling of brain cortex tissues in a Tau transgenic mouse model of Alzheimer’s disease. Biochem Biophys Res Commun 430:670–675.


Chatterjee P, Bhattacharyya M, Bandyopadhyay S, Roy D. 2014. Studying the system-level involvement of microRNAs in Parkinson’s disease. PLoS One 9:e93751



Cho HJ, Liu G, Jin SM, Parisiadou L, Xie C, Yu J, Sun L, Ma B, Ding J, Vancraenenbroeck R, Lobbestael E, Baekelandt V, Taymans JM, He P, Troncoso TC, Shen Y, Cai H. 2013. MicroRNA-205 regulates the expression of Parkinson’s disease-related leucine-rich repeat kinase 2 protein. Hum Mol Genet 22:608–620.



Cummings JL, Vinters HV, Cole GM, Khachaturian ZS. 1998. Alzheimer’s disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology 51:S2–17. discussion S65–7

Groenen MA, Archibald AL, Uenishi H, Tuggle CK, Takeuchi Y, Rothschild MF, Rogel-Gaillard C, Park C, Milan D, Megens HJ, et al. 2012. Analyses of pig genomes provide insight into porcine demography and evolution. Nature 491:393–398.



Head E. 2007. Combining an antioxidant-fortified diet with behavioral enrichment leads to cognitive improvement and reduced brain pathology in aging canines: strategies for healthy aging. Ann NY Acad Sci 1114:398–406.


Head E, Torp R. 2002. Insights into Abeta and presenilin from a canine model of human brain aging. Neurobiol Dis 9:1–10.


Johnstone EM, Chaney MO, Norris FH, Pascual R, Little SP. 1991. Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Mol Brain Res 10:299–305.


Kragh PM, Nielsen AL, Li J, Du Y, Lin L, Schmidt M, Bogh IB, Holm IE, Jakobsen JE, Johansen MG, Purup S, Bolund L, Vajta G, Jorgensen AL. 2009. Hemizygous minipigs produced by random gene insertion and handmade cloning express the Alzheimer’s disease-causing dominant mutation APPsw. Transgenic Res 18:545–558.


Li J, Wu Z, Cheng F, Li W, Liu G, Tang Y. 2014. Computational prediction of microRNA networks incorporating environmental toxicity and disease etiology. Sci Rep 4:5576



Lukiw WJ. 2007. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport 18:297–300.


Maciotta S, Meregalli M, Torrente Y. 2013. The involvement of microRNAs in neurodegenerative diseases. Front Cell Neurosci 7:265



Muller M, Kuiperij HB, Claassen JA, Kusters B, Verbeek MM. 2014. MicroRNAs in Alzheimer’s disease: differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiol Aging 35:152–158.


Peterson KJ, Dietrich MR, McPeek MA. 2009. MicroRNAs and metazoan macroevolution: insights into canalization, complexity, and the Cambrian explosion. Bioessays 31:736–747.


Ramanan VK, Saykin AJ. 2013. Pathways to neurodegeneration: mechanistic insights from GWAS in Alzheimer’s disease, Parkinson’s disease, and related disorders. Am J Neurodegener Dis 2:145–175.


Sarasa M, Pesini P. 2009. Natural non-trasgenic animal models for research in Alzheimer’s disease. Curr Alzheimer Res 6:171–178.



Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504.



Shtilbans A, Henchcliffe C. 2012. Biomarkers in Parkinson’s disease: An update. Curr Opin Neurol 25:460–465.


Studzinski CM, Christie LA, Araujo JA, Burnham WM, Head E, Cotman CW, Milgram NW. 2006. Visuospatial function in the beagle dog: An early marker of cognitive decline in a model of human aging and dementia. Neurobiol Learn Mem 86:197–204.


Sutherland GT, Matigian NA, Chalk AM, Anderson MJ, Silburn PA, Mackay-Sim A, Wells CA, Mellick GD. 2009. A cross-study transcriptional analysis of Parkinson’s disease. PLoS One 4:e4955



Tan CL, Plotkin JL, Veno MT, von Schimmelmann M, Feinberg P, Mann S, Handler A, Kjems J, Surmeier DJ, O’Carroll D, Greengard P, Schaefer A. 2013. MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science 342:1254–1258.



Tiribuzi R, Crispoltoni L, Porcellati S, Di Lullo M, Florenzano F, Pirro M, Bagaglia F, Kawarai T, Zampolini M, Orlacchio A, Orlacchio A. 2014. miR128 up-regulation correlates with impaired amyloid beta(1–42) degradation in monocytes from patients with sporadic Alzheimer’s disease. Neurobiol Aging 35:345–56.


Vasudevan S, Tong Y, Steitz JA. 2007. Switching from repression to activation: microRNAs can up-regulate translation. Science 318:1931–1934.


Villa C, Ridolfi E, Fenoglio C, Ghezzi L, Vimercati R, Clerici F, Marcone A, Gallone S, Serpente M, Cantoni C, Bonsi R, Cioffi S, Cappa S, Franceschi M, Rainero I, Mariani C, Scarpini E, Galimberti D. 2013. Expression of the transcription factor Sp1 and its regulatory hsa-miR-29b in peripheral blood mononuclear cells from patients with Alzheimer’s disease. J Alzheimers Dis 35:487–494.


- TOOLS





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print





