Brunmeir R, Lagger S, Seiser C. 2009. Histone deacetylase 1 and 2-controlled embryonic development and cell differentiation. Int J Dev Biol 53:275ŌĆō289.


Galletti M, Cantoni S, Zambelli F, Valente S, Palazzini M, Manes A, Pasquinelli G, Mai A, Gali├© N, Ventura C. 2014. Dissecting histone deacetylase role in pulmonary arterial smooth muscle cell proliferation and migration. Biochem Pharmacol 91:181ŌĆō190.


Halevy O, Piestun Y, Allouh MZ, Rosser BW, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. 2004. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn 231:489ŌĆō502.


Jacob C, Christen CN, Pereira JA, Somandin C, Baggiolini A, L├Čtscher P, ├¢z├¦elik M, Tricaud N, Meijer D, Yamaguchi T, Matthias P, Suter U. 2011. HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nat Neurosci 14:429ŌĆō436.


Lamey TM, Koenders A, Ziman M. 2004. Pax genes in myogenesis: Alternate transcripts add complexity. Histol Histopathol 19:1289ŌĆō1300.

Lin W, Hashimoto SI, Seo H, Shibata T, Ohta K. 2008. Modulation of immunoglobulin gene conversion frequency and distribution by the histone deacetylase HDAC2 in chicken DT40. Genes Cells 13:255ŌĆō268.


Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2
ŌłÆ╬ö ╬öCT method. Methods 25:402ŌĆō408.


McKinsey TA, Zhang CL, Olson EN. 2001. Control of muscle development by dueling HATs and HDACs. Curr Opin Genet Dev 11:497ŌĆō504.


Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J, Qi X, Hill JA, Richardson JA, Olson EN. 2007. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev 21:1790ŌĆō1802.



Niegisch G, Knievel J, Koch A, Hader C, Fischer U, Albers P, Schulz WA. 2013. Changes in histone deacetylase (HDAC) expression patterns and activity of HDAC inhibitors in urothelial cancers. Urol Oncol-Semin Orig Investig 31:1770ŌĆō1779.

Rehfeldt C, Te Pas MFW, Wimmers K, Brameld JM, Nissen PM, Berri C, Valente LMP, Power DM, Picard B, Stickland NC, Oksbjerg N. 2011. Advances in research on the prenatal development of skeletal muscle in animals in relation to the quality of muscle-based food. 1. Regulation of myogenesis and environmental impact. Animal 5:703ŌĆō717.


Taunton J, Hassig CA, Schreiber SL. 1996. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272:408ŌĆō411.


Velleman SG. 2007. Muscle development in the embryo and hatchling. Poult Sci 86:1050ŌĆō1054.


Yoo JYJ, Larouche M, Goldowitz D. 2013. The Expression of HDAC1 and HDAC2 during cerebellar cortical development. Cerebellum 12:534ŌĆō546.


Zhu W. 2010. Mechanisms of Hdac2 Function in the Regulation of Adult Cardiac Hypertrophy and Embryonic Myocyte Proliferation. PhD Thesis. University of Pennsylvania; Philadelphia, PA, USA:
 is the effect of j-th treatment in i-th level, ╬╝ is the general mean,
is the effect of j-th treatment in i-th level, ╬╝ is the general mean,
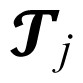 represents the effect of j-th treatment and Ōä░ij means random error due to i-th level of the j-th treatments.
represents the effect of j-th treatment and Ōä░ij means random error due to i-th level of the j-th treatments.




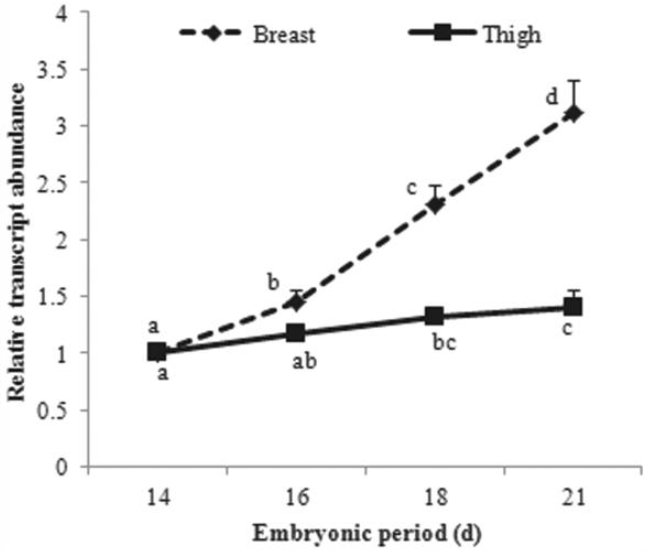
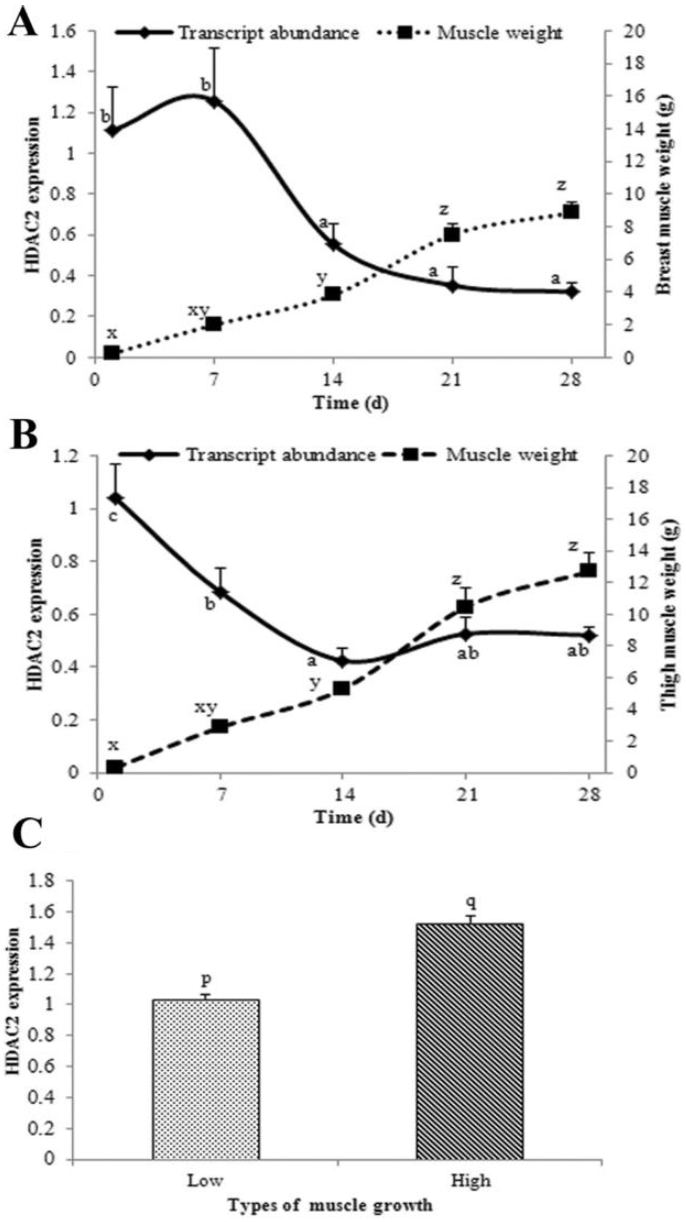
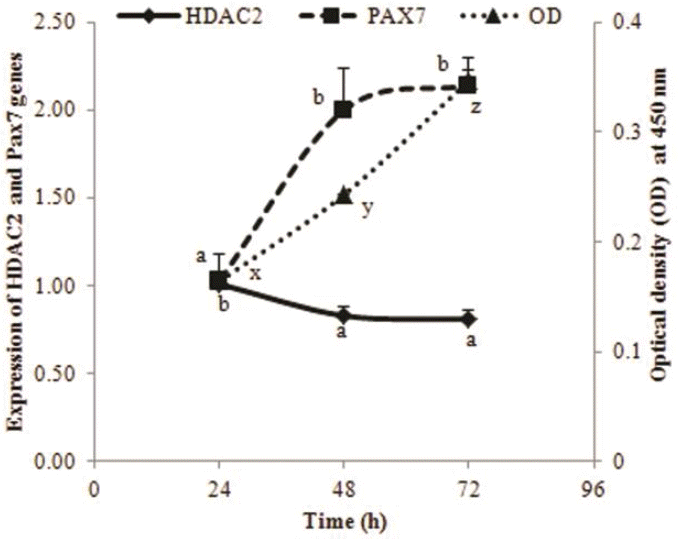
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print







