Lipid Sources with Different Fatty Acid Profile Alters the Fatty Acid Profile and Quality of Beef from Confined Nellore Steers
Article information
Abstract
The present study was conducted to determine the effects of lipid sources with different fatty acids profile on meat fatty acids profile and beef quality traits of Nellore. A total of 45 Nellore animals with an average initial body weight of 419±11 kg (at 15±2 mo) were distributed in a completely randomized design consisting of 5 treatments and 9 replicates. The roughage feed was maize silage (600 g/kg on a dry matter [DM] basis) plus concentrate (400 g/kg on a DM basis). The dietary treatments were as follows: without fat (WF), palm oil (PO), linseed oil (LO), protected fat (PF), and soybean grains (SG). No effects of lipid sources were observed (p>0.05) on beef color, pH, water-holding capacity, and sarcomere length. Beef from cattle fed PO had greater shear-force values (p<0.05) compared to beef from cattle fed WF. Deposition of main unsaturated fatty acids (oleic, linoleic, and linolenic) was greater in treatments WF, SG, and LO, respectively, while the values of conjugated linoleic acid (CLA) were greater when animals were fed LO. The inclusion of LO in the diet enhances the concentration of CLA in longissimus muscle and subcutaneous fat besides improving the atherogenicity index and elongase activity. As such, LO can be used with the aim to improve the quality of beef from confined Nellore cattle. Conversely, the use of PO is not recommended since it may increase the concentration of undesirable unsaturated fatty acids in muscle and subcutaneous fat, shear-force and the atherogenicity index.
INTRODUCTION
Modification of the fatty acids profile of meat to obtain a lower proportion of saturated fatty acids (SFA) is a reliable method to produce a healthier meat for consumers (Ladeira et al., 2014). One of the main strategies to improve the quality of final products of ruminant animals (milk and meat) is the ruminal manipulation with addition of lipid sources (Daley et al., 2010; Moloney et al., 2012). Feedstuff with different fatty acids profile may be used as lipid sources in diets of ruminant animals (Fiorentini et al., 2012; Oliveira et al., 2012; Ladeira et al., 2014). However a limitation for the deposition of unsaturated fatty acids (UFA) in the meat and dairy products of ruminants occurs due to ruminal biohydrogenation of UFA (Herdmann et al., 2010). According to Allen (2000), the biohydrogenation process is enhanced (up to 90%) and animals are fed diets containing oil as a lipid source.
An important lipid source is the rumen protected fat (PF) from soybean oil, which exhibit low fatty acid release in the rumen (Jenkins and Bridges, 2007), and may result in increased monounsaturated fatty acids (MUFA) deposition in meat. Oleaginous seeds such as soybean grains are also a potential source of slow release oil in the ruminal environment. Thus, the use of these sources may be a nutritional strategy that increases the ruminal escape of UFA (Duckett and Gillis, 2010), reducing the deposition of saturated fatty acids and enhancing the levels of UFA in animal tissues.
However, a limited number of studies have shown the effects of different dietary lipid sources on fatty acids profile and quality traits of meat from Zebu cattle during the finishing period. As such, the present study aimed to evaluate the qualitative traits, composition and quality of fatty acids of meat and subcutaneous fat from Nellore steers fed lipid sources with different fatty acids profile.
MATERIALS AND METHODS
The protocol used in this experiment was in accordance with the Brazilian College of Animal Experimentation (Colégio Brasileiro de Experimentação Animal, COBEA) guidelines and was approved by the Ethics, Bioethics, and Animal Welfare Committee (Comissão de Ética e Bem Estar Animal, CEBEA) of the FCAV, UNESP, Jaboticabal campus (protocol number 012799).
Animals and feeding
A total of 45 Nellore steers (initial body weight = 419±11 kg; 15±2 mo) were housed in individual 8 m2 (4×2 m) pens that were provided with a concrete trough and drinker. The animals spent 28 d acclimating to the diets, facilities, and management. After this period, they were confined for 90 d (experimental period).
The feeds were formulated to be isonitrogenous according to the NRC (2000). Five concentrates were formulated: i) without additional fat (WF; 27.9 g/kg of ether extract [EE] in the total diet); ii) with palm oil (PO) derived from the palmaceae plant Orbignya oleifera, which has a lipid profile rich in medium-chain fatty acids (lauristic and myristic); iii) with linseed oil (LO); iv) with PF, Lactoplus - Dalquim group, Itajaí, Santa Catarina, Brazil; and v) with soybeans grains (SG). All the diets with added lipids contained an average of 42 g of additional lipid per kg of dry matter (DM).
The roughage was corn silage (600 g/kg on a DM basis and with the following composition: 369 g DM/kg, 71 g crude protein/kg DM, 477 g neutral detergent fiber/kg DM, 23 g EE/kg DM, and 53 g lignin/kg DM) and concentrate (400 g/kg on a DM basis). The concentrates were mixed weekly to restrict oxidation and rancidity of ingredients. The lipid sources (PO, LO, PF, and SG) were incorporated into the remaining ingredients of the concentrate in a horizontal mixer for 15 min. The proportions of ingredients and the chemical composition of the experimental diets are presented in Table 1. The animals were fed with corn silage and the experimental concentrates once a day at 08:00 h as a total mixed ration. Throughout the entire experimental period, the provided quantities were adjusted to allow a surplus of approximately 100 g/kg in relation to the total amount consumed on the previous day.
Chemical analyses of feedstuff
Samples of corn silage and concentrates were analyzed to determine DM (method 934.01) and mineral matter (method 942.05) and to obtain an acid EE (method 954.02) according to AOAC (1990). Nitrogen was determined using an LECO FP-528 nitrogen analyzer (LECO Corp., St. Joseph, MI, USA). Neutral detergent fiber was determined using α-amylase and without the addition of sodium sulfite following Van Soest et al. (1991) and adapted for the Ankom200 Fiber Analyzer (Ankom Technology, Fairport, NY, USA). Acid detergent fiber was determined using the method described by Goering and Van Soest (1970) and adapted for the Ankom200 Fiber Analyzer (Ankom Technology, USA).
To determine the fatty acid composition of feed offerings, a sample of approximately 1 g was used. The frozen sample was homogenized in 20 mL of a chloroform and methanol solution (2:1) using a Turrax homogenizer, disintegrator, and emulsifier (Folch et al., 1957). In the next step, the lipid extract aliquot was methylated using the protocol in Kramer et al. (1997). Fatty acids were quantified using a GC 2010 gas chromatograph (Shimadzu Corp., Kyoto, Japan) with an SP-2560 capillary column (100 m×0.20 mm i.d. with a 0.02-μm film thickness; Supelco, Bellefonte, PA, USA). The initial temperature was set to 70°C for 4 min (13°C/min) until it reached 175°C and then held for 27 min. After this, the temperature was increased 4°C/min until it reached 215°C and was held there for 31 min. Hydrogen was used as the carrier gas with a flow of 40 cm3/s (Table 1).
Slaughter, carcass data collection and meat sampling
At the end of the experimental period cattle were transported to a commercial beef slaughterhouse and slaughtered at the same day. Pre-harvest handling was in accordance with good animal welfare practices, and slaughtering procedures followed the Sanitary and Industrial Inspection Regulation for Animal Origin Products. After the slaughter carcasses were chilled for 24 hours in cold chamber. After the chilling period meat samples were collected though a perpendicular cut in the longissimus muscle between the 12th and 13th ribs the right half of the carcass. Longissimus muscle samples were individually vacuum-packaged and held at −20°C for 2 days. Each frozen longissimus sample was standardized from the posterior end into one 2.54 cm thick steak sample (AMSA, 1995) for Warner-Bratzler shear force measurement and two 1 cm thick steaks for further analysis. All steaks were vacuum packaged and held at −20°C for 10 days until the analysis were performed.
Meat and subcutaneous fat instrumental color analysis
Meat and fat instrumental color analysis was performed as described by Houben et al. (2000) using a Minolta colorimeter (Model CR 300, Minolta Camera Co. Ltd., Osaka, Japan) to evaluate lightness (L*), redness (a*), and yellowness (b*). The color aspects were assessed by the Commission Internationale de l’Eclairage L*a*b* color system using 0°/45°. Samples of meat and subcutaneous fat were allowed to bloom for 30 min prior the color evaluation. After this step objective color was measured at 3 locations on the displayed surface of each steak and the average values of L* a* b* were calculated. The colorimeter was calibrated according to the manufacturer before analyzing the samples.
The chroma (C) and hue (h) values were calculated as described by MacDougal (1994) by using the values of lightness (L*), redness (a*), and yellowness (b*) obtained from the color measurements as it follows:
Warner–Bratzler shear force measurement and cooking losses
Warner–Bratzler shear force (WBSF) steaks were thawed at 4°C for 24 h and oven-broiled in an electric oven (Layr, Luxo, Inox) preheated to 150°C. Internal steak temperatures were monitored by 20-gauge copper-constantan thermocouples (Omega Engineering, Stamford, CT, USA) placed in the approximate geometric center of each steak and attached to a digital monitor. When internal steak temperature reached 35°C, the steak was turned over and allowed to reach an internal temperature of 70°C before removal from the oven. Cooked WBSF steaks were cooled for 24 h at 4°C (AMSA, 1995). Eight round cores (1.27 cm diameter) were removed from each steak parallel to the long axis of the muscle fibers (AMSA, 1995). Each core was sheared once through the center, perpendicular to the fiber direction by a Warner-Bratzler shear machine (G-R Manufacturing Company, Manhattan, KS, USA). The cooking losses were evaluated on the steaks that were also used for WBSF measurement. Total cooking loss was calculated as the difference between the weight of the steaks before and after oven-broiling.
Sarcomere length
Longissimus muscle samples were collected at 24 h postmortem and small cubes (3.0×3.0×2.0 cm) were excised in triplicate from each sample. Cubes were then fixed as described by Koolmees et al. (1986). Sarcomere length was measured by laser diffraction using a 05-LHR-021 laser, Melles Griot (Carlsbad, CA, USA) and calculated as described by Cross et al. (1981). From each cube, sarcomere length of six fiber samples was determined and used for sarcomere length average calculation.
Fatty acid profile
To determine the fatty acid composition of the fresh meat samples the transversal section were collected from the longissimus muscle, freeze-dried, and frozen for lipid extraction and methylation. The fatty material was extracted using a mixture of chloroform–methanol as reported by Bligh and Dyer (1959) and the fatty acids methyl esters were obtained by ISO 5509 method (ISO 5509, 2000). Qualitative and quantitative measurements of fatty acid content were performed by gas chromatography using a chromatographer (Model GC-14B with a Communication Bus Module, CBM 102; Shimadzu, Kyoto, Japan) with a flame ionization detector and fused silica capillary column (Omegawax 250), which was 30 m length and 0.25 mm diameter and had a film thickness of 0.25 μm (Supelco SP-24136, USA). Helium was used as a carrier gas at a flow of 1 mL/min. A 1-μL aliquot of the sample was injected into a “split” at a division ratio of 1/100 and a temperature of 250°C. The temperature of the oven was programmed to remain at 100°C for 2 min and then increased to 220°C at 4°C/min for 25 min, while the detector was at 280°C. Identification and quantification of the methyl esters of the fatty acids was achieved by comparison with the retention times and concentrations of methyl esters of standard fatty acids.
The activity index were calculated for elongase and Δ9-desaturase enzymes on fatty acids with 16 and 18 carbons which are responsible for the conversion of SFA with 16 and 18 carbons into their respective correspondents monounsaturated with double bond in carbon 9 as described by MalauAduli et al. (1997). The atherogenecity index was calculated as described by Ulbricht and Southgate (1991) as an indicator of risk of cardiovascular disease. Calculations were performed as it follows:
Statistical analysis
The statistical analysis was conducted using the general linear model procedure of SAS (Statistical Analysis System, version 9.1) as the following model:
where Yij = observation of animal j subject to treatment i; μ = the overall mean; ti = effect of treatment i; i = 1; ... 5; and eij = the residual experimental error. Least square means were compared using Tukey’s method at α = 0.05.
RESULTS
There was no effect of lipid sources on pH (p = 0.533) and color measurements of longissimus muscle and subcutaneous fat (Table 2). The water-holding capacity (p = 0.671) and sarcomere length (p = 0.271) were not affected by lipid sources. However, differences were observed for WBSF (p = 0.011) and cooking losses (p = 0.011) of beef from cattle fed different lipid sources. Beef from animals fed PO had greater values of shear force and cooking losses than beef from animals fed diets without addition of lipid source (Table 3).

Instrumental color evaluation of meat and subcutaneous fat, and final pH of confined Nellore steers fed diets with different lipid sources
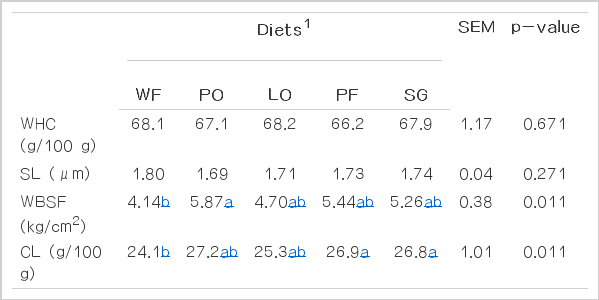
Water holding capacity (WHC), sarcomere length (SL), Warner–Bratzler shear force (WBSF), and cooking losses (CL) of longissimus muscle from confined Nellore steers fed diets with different lipid sources
With regard to fatty acid profile, with exception of C17:0 (margaric) in longissimus muscle and subcutaneous fat and C18:0 (stearic acid) in the subcutaneous fat, all the fatty acids were affected (p<0.05) by lipid sources (Table 4). Animals fed PO had greater concentration of SFA C12:0 (lauric), C14:0 (myristic) and C15:0 (pentadecanoic) in both meat and subcutaneous fat (p<0.001).
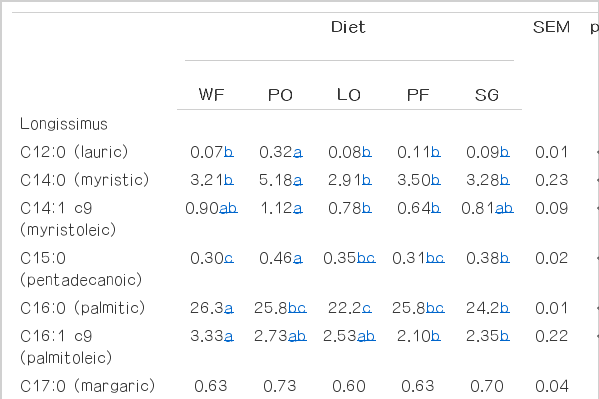
Main fatty acids (g/100 g of total fatty acids) of longissimus muscle and subcutaneous fat from confined Nellore steers fed diets with different lipid sources
The concentration of C16:0 (palmitic) was greater in meat of animals fed diets without addition of lipid source and lower in meat from animals fed LO (p<0.011). On the other hand, the lowest concentration of palmitic acid was observed in subcutaneous fat of animals fed LO (p<0.001).
The lowest concentration of C18:0 was observed in meat from animals fed diets without addition of lipid source when compared to diets containing PF and PO (p = 0.011). The addition of lipid sources reduced the concentration of oleic acid in meat (p<0.001). However, in subcutaneous fat the reduction in the oleic acid concentration was observed only in animals fed PO and PF (p<0.001).
A greater percentage of linoleic acid in the longissimus muscle and subcutaneous fat were observed in animals fed diets containing PF (p<0.001). The use of LO enhanced the concentration of alpha-linolenic acid and conjugated linoleic acid (CLA) (18:2 c9, t11) in longissimus muscle and subcutaneous fat (p<0.011).
Animals fed PO and PF had greater concentrations of SFA in longissimus muscle (p<0.001; Table 5). A greater concentration of SFA in subcutaneous fat was observed in animals fed diets containing PO. The concentration of MUFA in both longissimus muscle and subcutaneous fat was lower for animals fed diets containing PO and PF (p<0.001).

Main fatty acids (g/100 g of total fatty acids) of longissimus muscle and subcutaneous fat from confined Nellore steers fed diets with different lipid sources
Cattle fed PF had the greatest concentration of polyunsaturated fatty acids (PUFA) being similar to those observed for animals fed LO and soybean oil for longissimus muscle composition (p<0.011), and LO for composition of subcutaneous fat. The concentrations of UFA and the UFA:SFA ratio were lower in longissimus muscle and subcutaneous fat of animals fed PO and did not differ from longissimus muscle of animals fed PF.
Longissimus muscle and subcutaneous fat from animals fed diets containing PF had greater PUFA:SFA ratio than those fed diets containing PO. However, similar values of PUFA:SFA ratio was observed in subcutaneous fat of animals fed diets containing PF and LO.
Longissimus muscle and subcutaneous fat from animals fed diets containing PF and LO had greater values of total trans18 fatty acids (p<0.011). A greater amount f total cis18 fatty acids was observed on longissimus muscle and subcutaneous fat of animals fed diets without addition of lipid source, LO and soybean grains (p<0.001).
A greater concentration of fatty acids of ω-6 family was observed on muscle and subcutaneous fat of cattle fed PF (p<0.001). The addition of LO in the diet increased the concentration of fatty acids from ω-3 family in muscle ans subcutaneous fat.
The C16 and C18 Δ9-desaturase activity was greater in longissimus muscle from animals fed diets without addition of lipid source and lower in longissimus muscle of animals fed diets with PF (p<0.011). The activity of C18 Δ9-desaturase in muscle of animals fed PO was lower when compared to the muscle of animals that did not receive lipid supplementation. A greater activity of C16 Δ9-desaturase was observed in tissues of animals fed diets with LO compared to the tissues of animals fed diets with PF (p = 0.031). On the other hand, the C18 Δ9-desaturase activity was greater in subcutaneous tisse of animals fed diets without addition of lipids compared to those fed diets with PO and PF (p = 0.011; Table 6).
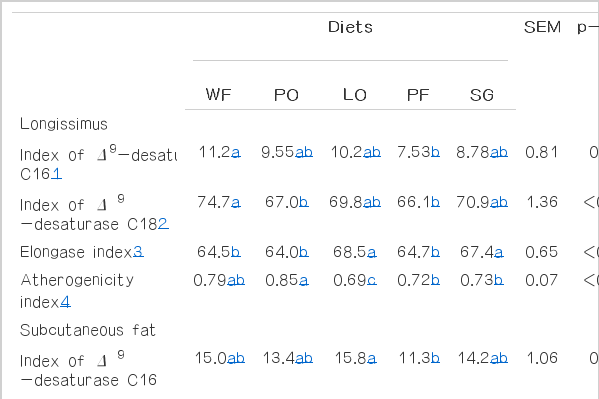
Index of enzymes involved on fatty acids metabolism, atherogenicity and elongase index in longissimus muscle and subcutaneous fat from confined Nellore steers fed diets with different lipid sources
A lower atherogenicity index was observed in longissimus muscle and subcutaneous fat of animals fed diets containing LO (p<0.001). Animals fed LO and soybean grains had the greatest elongase activity in longissimus muscle and subcutaneous fat (p<0.001).
DISCUSSION
The pH values observed in the current study are within the normal pH range for beef (5.4 to 5.8) (Mach et al., 2008). The final pH is dependent on the acid lactic accumulation as a result of adenosine triphosphate production by using glycogen as a source of glucose (Oliveira et al., 2011). In general, cattle fed grain based diets have greater availability of glycogen at slaughter and lower ultimate pH of meat (Neath et al., 2007). Final pH values suggests that there was no pre-slaughter stress since muscle acidification has occurred as expected, and that final pH was not altered by the lipid sources evaluated. According to Fernandes et al. (2008) the beef industry in Brazil only exports meat with pH values lower than 5.8, evaluated 24 h postmortem directly on longissimus muscle.
The lack of effects of lipid sources on beef color may have occurred as a consequence of the lack of differences on pH values. The color of meat and fat is an important trait that influences the consumer’s choice at purchase (Page et al., 2001). Muchenje et al. (2009) reported the following ranges for L*, a*, and b* values in beef: 33 to 41, 11.1 to 23.6, and 6.1 to 11.3, respectively. In the current study the values of b* were lower than those values. Oliveira et al. (2012) in a study with inclusion of soybean oil, LO, and calcium salt of soybean and LO in diets of confined young Nellore steers reported similar average values of those observed in the present study (L* = 38.3; a* = 16.8; b* = 6.46) probably because the similarity of the animals used in both studies which had the same age and same breed.
The cold shortening may decrease meat tenderness and thus compromises its quality (Savell et al., 2005). According to Bouton et al. (1973), WBSF decreases exponentially as the sarcomere length increases but this correlation significant only for length lower than 2.0 μm. In the present study the average value of sarcomere length was 1.74 μm. However, the sarcomere length did not affect the WBSF of longissimus muscle.
Animals fed WF diet had lower values of cooking losses which was the main factor that affect meat WBSF observed for these animals. According to Belew et al. (2003) meat is considered extremely tender when shear force values is lower than 3.2 kg/cm2; tender when WBSF values are within 3.2 and 3.9 kg/cm2; intermediate tenderness when WBSF values are within 3.9 and 4.6 kg/cm2, and tough when WBSF values are greater than 4.6 kg/cm2. Therefore, in the current study only animals fed diets without addition of lipid sources can be classified as “intermediate tenderness” while meat from the animals fed the other diets can be classified as “tough”. Oliveira et al. (2012) did not find effect of supplementation with lipid source on WBSF of meat from confined young Nellore steers and reported values slightly higher (5.97 kg/cm2) than those found the current study.
Another factor that may have contributed to the high values of WBSF observed in this study is the use of Bos indicus animals. It is well documented that the mean WBSF and variation in shear force increases as the percentage of Bos indicus, such as Nellore, inheritance increases (Lage et al., 2012). This is due to the fact that a greater calpastatin activity is commonly observed in Zebu cattle, which inhibits calpain activity leading to a less tenderization of beef (Curi et al., 2009).
The higher concentration of saturated fatty acids in animals fed PO was likely due to the saturated fatty acid profile of this lipid source compared to the other diets (Table 1). As reported by Scollan et al. (2006), the most saturated fatty acids found in beef are C14:0, C16:0, C18:0 (stearic), and the C18:0 represents approximately 30% of the total saturated fatty acids. Among these fatty acids, the myristic and palmitic acids are of importance as they are considered hypercholesteromic (Wood et al., 2003). As reported by Oliveira et al. (2012), the concentration of C14:0 has a potential four times greater than C16:0 to increase the plasmatic concentration of cholesterol.
There is a correlation among C18 Δ9-desaturase and the concentration of oleic acid (Oliveira et al., 2011), which was confirmed in the current study as a highest concentration of oleic acid was observed in animals fed diets without addition of lipid source and lowest concentration was observed in animals fed diets with PO and PF. The greater concentration of linolenic acid in animals fed diets with PF was due to the greater level of this acid in the diet (Table 1). As the PF is partially inert in the ruminal environment, less biohydrogenation is observed in these acids in the rumen (Fiorentini, 2013). Consequently, a greater deposition of linoleic acid may occur in muscle and subcutaneous fat. Regarding the higher concentration of linolenic acid in muscle of animals fed LO and PF, it can be attributed to the greater amount of this acid in the diet which lead to a greater intake of this acid by the animals.
The diet with LO increased the levels of CLA (18:2 c9 t11) in muscle and subcutaneous fat being in accordance with results previously reported by Oliveira et al. (2012) who observed values of CLA (0.72 g/100 g) close to those found in the present study (0.67 g/100 g). The increase of CLA amount in the carcass of animals fed LO is due to the amount of linolenic acid found in such source (Table 1). Before the biohydrogenation, the triglycerides that reach the ruminal environment are hydrolyzed into fatty acids and glycerol. During the biohydrogenation process, isomeriation reactions convert cis bounds into trans bounds before the beginning of the reduction reactions which add hydrogen to the double bounds. However, not all the UFA that reach the ruminal environment are biohydrogenated in this process and part of the intermediate UFA with trans bounds flows to the duodenum and then deposited in the tissues (Duckett and Gillis, 2010). Thus, a greater amount of intermediate fatty acids escapes from the ruminal biohydrogenation as the dietary substrates for biohydrogenation (C18:2 and C18:3) increases, leading to an enhancement of CLA accumulation in the carcass.
Besides the CLA that escapes the ruminal biohydrogenation, the vaccenic acid (trans C18:1) may also be converted into CLA in the adipose and muscle tissues though the activity of desaturase enzyme (C18-Δ9) in both animals and humans. Diets with a potential to elevate the concentration of CLA such as soybean grains (slow released of oil in the rumen), likely did not released enough amounts of linoleic or linolenic acids for biohydrogenation, as reported by Nelson et al. (2008) in a study with vegetal oil.
The C16 Δ9-desaturase is responsible for the desaturation of saturated fatty acids with 16 and 18 carbons and their conversion into their correspondent monounsaturated with a double bound on carbon 9. This was observed in the present study since diets with PF had lower concentration of MUFA. The different level of palmitoleic acid found in the longissimus muscle was due to the activity of C16 Δ9-desaturase which is responsible for the conversion of palmitic into palmitoleic acid (Oliveira et al., 2011).
A greater intake of MUFA is beneficial to human health as it decreases the levels of plasmatic total cholesterol (Department of Health of London, 1994). In the present study it is represented by animals fed diets without addition of lipid source, LO and soybean grains, likely due to the combination of lipid profile of the diets in association of the ingestion of these acids.
According to Sanhueza et al. (2002), differently than UFA cis, the trans fatty acids have on the hydrogen molecules of their double bound in a transversal disposition being a result of ruminal biohydrogenation or from industrial process. The general concept of trans fatty acids refers to a nonbeneficial effects on human health with blood lipid effects, inhibition of hepatic enzymes, challenge on fluidity of cell membrane, and arteriogenic potential, with exception of the endogenous precursor of CLA, the CLA and its isomers which have benefic effects related to immunoestimulating, antimutagenesis, and antioxidant activities (Ip, 1997). Muscle of animals fed LO and PF had higher concentration of trans fatty acids likely due to the combination of composition and diet intake. However, besides this observation, it cannot be affirmed that theses lipid sources should not be used because of its nonbeneficial effects since the composition of other fatty acids must be evaluated.
The LO had the lowest atherogenicity index, showing a great potential to improve the quality of meat and subcutaneous fat. This is an important trait as it is related to pro and anti-atherogenic acids and indicates the potential stimuli to platelets aggregation. Therefore the amount of anti-atheorgenic fatty acids in the subcutaneous fat is increased by the enhancement of the atherogenicity index. Consequently, there is a greater potential to prevent the occurrence of coronary artery diseases (Ulbricht and Southgate, 1991).
According to Lopes et al. (2012) there is a correlation among the concentration of palmitic acid, palmitoleic acid and enhancement of oleic acid. Thus, the higher activity of elongase enzyme observed in animals fed LO and soybean grains may be explained by a lower concentration of palmitic and palmitoleic acids in addition to a greater amount of oleic acid. According to Smith et al. (1995), elongase enzyme has higher activity in adipose than muscle tissue, which was not observed in the present study.
The fatty acids from ω-3 and ω-6 families are essential for humans as it has not an endogenous synthesis. Thus, the ω-3 intake is encouraged and the ω-6:ω-3 ratio is recommended to be lower than 4:1 (Wood et al., 2003), and higher values may indicate a non-healthy diet that may lead to a coronary artery diseases (Department of Health of London, 1994). The ω-6:ω-3 ratio observed in meat of animals fed diets without addition of lipid sources, with palm or LO was closer of the recommended value. Conversely, the values of ω-6:ω-3 ration observed in the subcutaneous fat were higher than the recommended values (4:1) for all treatments.
CONCLUSION
The addition of lipid sources in finishing diets of confined young steers alters longissimus muscle quality the profile of fatty acids. The inclusion of LO in the diet enhances the concentration of CLA in longissimus muscle and subcutaneous fat besides the improvement of atherogenicity index and elongase activity. As such, LO can be used with the aim to improve the quality of beef from confined Nellore cattle. Conversely, the use of PO is not recommended since it may increase the concentration of undesirable UFA in muscle and subcutaneous fat, shear-force and the atherogenicity index.
ACKNOWLEDGMENTS
We thank the São Paulo Research Foundation (FAPESP) (grants #2009/ 51215-1 and 2009/ 06472-6) and Bellman Nutrição Animal for providing financial support. We thank all of the participants for their help in the study. Giovani Fiorentini is the main author. Study is a part of my doctoral thesis. I participated in all the processes of the study, from designing to conducting the experiment, laboratory analysis, statistical analysis and drafted the manuscript. Josiane F. Lage and Isabela P. C. Carvalho, are doctoral colleagues which had their thesis from the same experiment. They participated in the study design, in the conduction of the experiment and laboratory analyzes. Juliana D. Messana and Roberta C. Canesin, are post-doctoral at the São Paulo State University who interpretation of data, the drafting the article or revising it critically for important intellectual content. Ricardo A. Reis is teacher and research in School of Agricultural Sciences. Participated in planning the study as co-advisor and assisted discussion of the results. Telma T. Berchielli is my advisor of my thesis. She participated in the study design, statistical analysis and discussion of the results. The authors declare that there is no conflict of interests.
