Effects of Aspergillus Oryzae Culture and 2-Hydroxy-4-(Methylthio)-Butanoic Acid on In vitro Rumen Fermentation and Microbial Populations between Different Roughage Sources
Article information
Abstract
An in vitro experiment was conducted to evaluate the effects of Aspergillus oryzae culture (AOC) and 2-hydroxy-4-(methylthio)-butanoic acid (HMB) on rumen fermentation and microbial populations between different roughage sources. Two roughage sources (Chinese wild rye [CWR] vs corn silage [CS]) were assigned in a 2×3 factorial arrangement with HMB (0 or 15 mg) and AOC (0, 3, or 6 mg). Gas production (GP), microbial protein (MCP) and total volatile fatty acid (VFA) were increased in response to addition of HMB and AOC (p<0.01) for the two roughages. The HMB and AOC showed inconsistent effects on ammonia-N with different substrates. For CWR, neither HMB nor AOC had significant effect on molar proportion of individual VFA. For CS, acetate was increased (p = 0.02) and butyrate was decreased (p<0.01) by adding HMB and AOC. Increase of propionate was only occurred with AOC (p<0.01). Populations of protozoa (p≤0.03) and fungi (p≤0.02) of CWR were differently influenced by HMB and AOC. Percentages of F. succinogenes, R. albus, and R. flavefaciens (p<0.01) increased when AOC was added to CWR. For CS, HMB decreased the protozoa population (p = 0.01) and increased the populations of F. succinogenes and R. albus (p≤0.03). Populations of fungi, F. succinogenes (p = 0.02) and R. flavefacien (p = 0.03) were increased by adding AOC. The HMB×AOC interactions were noted in MCP, fungi and R. flavefacien for CWR and GP, ammonia-N, MCP, total VFA, propionate, acetate/propionate (A/P) and R. albus for CS. It is inferred that addition of HMB and AOC could influence rumen fermentation of forages by increasing the number of rumen microbes.
INTRODUCTION
Chinese wild rye (CWR) and corn silage (CS) are major roughages which are commonly fed to cattle in China’s dairy farms. However, the conversion efficiency of roughages to meat and milk is limited by low digestibility of roughage cell walls and low yield of microbial protein in the rumen (Wang and McAllister, 2002). Therefore, it is important to improve fiber digestibility and microbial protein synthesis for achieving greater feed utilization and animal production. The use of Aspergillus oryzae culture (AOC) increased digestion of feedstuffs mainly through improved rumen fiber degradation (Chen et al., 2004; Di Francia et al., 2008). However, AOC had variable effect on different roughages, and had no significant improvement on CS (Beharka and Nagaraja, 1993).
Rumen-protected Met, 2-hydroxy-4-(methylthio)-butanoic acid (HMB) is a source of L-Met which is commonly used in ruminant feeds, because Met has been considered as the limiting amino acid for high producing dairy cattle diets (NRC, 2001). Nevertheless, HMB does not totally escape rumen microbial metabolism and can be a source of Met for rumen microorganisms. However, the impact of HMB on rumen fermentation seems highly variable and remains unclear. Although addition of HMB improved microbial protein synthesis (Vázquez-Añón et al., 2001), some researchers found no changes in volatile fatty acid (VFA) proportions by HMB (Vázquez-Añón et al., 2001; Wilson et al., 2008), whereas others reported changes in VFA profile (Noftsger et al., 2003; Chung et al., 2006). In addition, the mechanisms by which HMB could impact microbial digestion in the rumen are not yet clear.
Supplementing AOC can enhance utilization of fiber, and adding HMB probably supplies Met and balances the amino acids to improve protein utilization in the rumen. It is well-known that fiber and protein are very important nutrients for ruminants. The simultaneous enhanced utilization of fiber and protein with supplementation of AOC and HMB may achieve a synergistic improvement in rumen functions. So a trial was conducted in which both AOC and HMB were allocated simultaneously as supplementations to enhance rumen function. For this purpose, we used an in vitro experiment to evaluate the effects of AOC and HMB on rumen fermentation and microbial populations in two different roughage sources (CWR and CS).
MATERIALS AND METHODS
Experimental design
Two kinds of roughages (CWR vs CS) were used as roughage substrates. They were analyzed for dry matter (DM), crude protein (CP), ash (AOAC, 1997), neutral detergent fiber (NDF) and acid detergent fiber (ADF) expressed inclusive of residual ash (Van Soest et al., 1991). Chemical composition of the two roughages is presented in Table 1. Each roughage substrate was assigned to all the treatments in a 2×3 factorial arrangement with AOC and HMB as main effects. The AOC (Amaferm, Biozyme, Inc., St. Joseph, MO, USA), was added into substrates at the level of 0, 3, or 6 mg. The HMB (MFP, Novus Int. Inc. St. Louis, MO, USA), was added into substrates at the level of 0 or 15 mg together with AOC. The treatments included: control (no AOC or HMB), AOC group without HMB (3 mg or 6 mg), HMB group without AOC (15 mg) and AOC plus HMB group (3 mg AOC+15 mg HMB; 6 mg AOC+15 mg HMB). Each treatment was performed in triplicate.
In vitro gas test procedure
The substrates in the in vitro test consisted of finely ground corn (100 mg) and one kind of roughage (100 mg), CWR or CS. The gas production (GP) was determined according to the method of Menke and Steingass (1988). Rumen fluids were collected before morning feeding from three rumen-fistulated cows fed twice daily on a mixed diet. Ingredients and chemical composition (% DM) of the diet are shown in Table 2.
Rumen fluid was strained through four layers of gauze into a pre-warmed syringes flushed with CO2 at 39°C. Syringes were filled with 30 mL medium consisting of 10 mL rumen fluid and 20 mL artificial saliva. The syringes were incubated in a water bath (39°C) for 24 h. The GP in vitro was recorded at 3, 6, 9, 12, and 24 h incubation times.
Sampling and measurement
The incubation was stopped at 24 h fermentation and the pH of rumen liquor was determined immediately using a pH meter (S40 SevenMulti, Mettler Toledo, Shanghai, China). The incubated inoculants were used for analysis of ammonia-N, VFA, microbial protein (MCP) and microbial communities. Ammonia-N, VFA and MCP concentrations were determined using methods of Hu et al. (2005).
Total DNA was isolated using a genomic DNA kit (Axygen Biosciences, Union City, CA, USA) following the manufacturer’s instructions. The amplifying primer sets of total bacteria, protozoa, fungi, F. succinogenes, R. flavefaciens and R. albus were designed as described by Denman and McSweeney (2006), Denman et al. (2007), and Koike and Kobayashi (2001), respectively as in Table 3. The species-specific real-time qPCR was performed using the ABI 7500 real time PCR system (Applied Biosystems, Foster City, CA, USA) with fluorescence detection of SYBR green dye. Samples were run under the amplification conditions as described by Mao et al. (2010).
Calculations and statistical analysis
To describe the dynamics of GP over time, the following equation (Ørskov and McDonald, 1979) was chosen:
where, GP = cumulative GP (mL) at t time, t = incubation time (h), a = the gas production from immediately soluble fraction (mL), b = the gas production from immediately insoluble fraction (mL), (a+b) = potential GP (mL), c = rate constant of GP (%/h), and a, b and c are constants.
The following equation of Menke and Steingass (1988) was used to calculate the in vitro organic matter digestibility (IVOMD):
where, GP = cumulative GP (mL) at 24 h, CP = crude protein content of substrates (g/kg).
Populations of protozoa, fungi, F. succinogenes, R. albus, and R. flavefaciens, were expressed as a proportion of total rumen bacterial 16S rDNA according to the equation of Chen et al. (2008):
where, Ct represented threshold cycle.
All of the statistical analyses were conducted using the general linear models (GLM) procedures of SAS software (SAS Institute, 1999). Data from CWR and CS were analyzed separately as a completely randomized design with HMB, AOC and their interaction included. Effect of AOC supplementation was determined by the “contrast” option of the GLM procedure. When this effect was significant (p<0.05), orthogonal polynomial (ORPOLY) contrasts using contrast coefficients that were obtained by ORPOLY macro were used to determine linear and quadratic responses to the concentrations of the AOC treatment. Significance was declared at p<0.05.
RESULTS
In vitro gas production and microbial protein
In vitro rumen GP, ammonia-N and MCP at 24 h incubation with substrates of CWR and CS are given in Table 4.
Chinese wild rye
The addition of AOC and HMB had no significant effect on rumen pH (p>0.05). Supplementing HMB significantly increased GP, c, IVOMD and MCP (p< 0.01). Ammonia-N was markedly decreased by adding HMB (p = 0.02). Addition of AOC influenced GP, IVOMD and MCP (linear, p<0.01; quadratic, p<0.01) and c (linear, p<0.01). The HMB×AOC interactions were noted in MCP (p<0.01).
Corn silage
GP, IVOMD, and MCP were increased (p≤0.02) in response to HMB addition (Table 4). However, adding HMB decreased c and ammonia-N (p≤0.02). The GP and IVOMD (linear, p<0.01), c (linear, p<0.01; quadratic, p = 0.04) and MCP (linear, p<0.01; quadratic, p<0.01) were increased as AOC concentration increased. Ammonia-N was decreased in response to addition of HMB (p<0.01) and AOC (linear, p<0.01). The HMB×AOC interactions for GP, c, IVOMD, ammonia-N and MCP were observed (p≤0.02).
Volatile fatty acid
Effects of HMB and AOC on total VFA and molar proportion of each fatty acid of two forages are presented in Table 5.
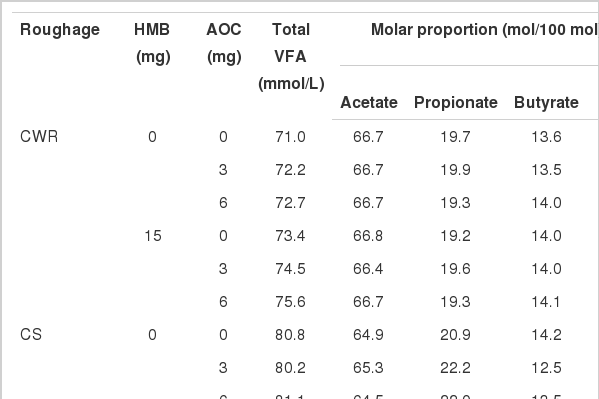
Effects of AOC and HMB on total VFA and individual VFA at 24 h incubation for different roughage sources
Chinese wild rye
Total VFA concentrations were significantly increased with the addition of HMB (p<0.01) and AOC (linear, p<0.01). Neither HMB nor AOC had a significant effect on molar proportions of acetate, propionate, butyrate and acetate/propionate (A/P) (p>0.05). There was no interaction for HMB and AOC on VFA (p>0.05).
Corn silage
Adding HMB significantly increased total VFA (p<0.01) and molar proportion of acetate (p<0.01), but decreased molar proportion of butyrate (p<0.01). Increases in total VFA (linear, p<0.01; quadratic, p<0.01), acetate (quadratic, p<0.01), propionate (linear, p = 0.04; quadratic, p = 0.02) and decreases in butyrate (quadratic, p<0.01) were noted as AOC concentration increased. The HMB×AOC interactions for total VFA, propionate, butyrate and A/P were observed (p≤0.04).
Rumen microbial population
Microbial populations (% of total bacterial 16S rDNA) of two forages are given in Table 6.
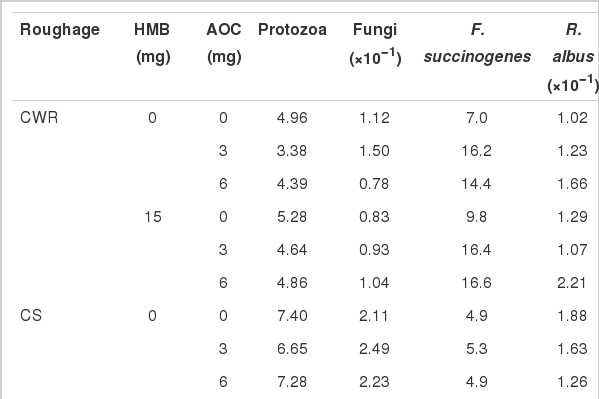
Effects of AOC and HMB on microbial populations (% of total bacterial 16S rDNA) for different roughage sources
Chinese wild rye
Adding HMB significantly increased protozoa percentage (p = 0.03) and decreased fungi percentage (p = 0.02) (Table 6). However, supplementing AOC significantly decreased protozoa percentage (quadratic, p = 0.01) and increased fungi percentage (quadratic, p<0.01). There was no significant effect of HMB on three kinds of fibrolytic bacteria (p>0.05). Percentages of F. succinogenes, R. albus (linear, p<0.01; quadratic, p≤0.02) and R. flavefaciens (linear, p<0.01) were increased in response to addition of AOC. There was an interaction between HMB and AOC for fungi and R. flavefaciens (p< 0.01).
Corn silage
Protozoa population was significantly decreased (p = 0.01), population of F. succinogenes and R. albus were markedly increased (p≤0.03) by adding HMB. Supplementing AOC significantly increased fungi (quadratic, p = 0.02), F. succinogenes (linear, p = 0.02) and R. flavefaciens (linear, p = 0.02). An interaction between HMB and AOC for R. albus was observed (p = 0.04).
DISCUSSION
In vitro gas production and microbial protein
The pH, GP, ammonia-N and MCP are important parameters reflecting ruminal environment. In the current study, terminal pH of two roughage sources was not influenced by HMB and AOC and all pHs were within the normal range. Addition of AOC and HMB caused marked increases of GP, IVOMD, and MCP which meant the two additives promoted rumen fermentations. The responses to AOC were very similar to the results of Lee et al. (2004) who found the addition of anaerobic fungal culture caused a marked increase in GP, cellulose digestion and bacterial population. There are few reports of HMB on GP in the in vitro test. In addition, for CS there were HMB×AOC interactions in GP and IVOMD which meant that the two additives interacted with each other and showed that adding HMB and AOC simultaneously to CS had a significant effect on GP and IVOMD.
Ammonia concentration in the rumen is a balance between degradation of feed protein and uptake of ammonia for synthesis of microbial protein. Ammonia-N decreasing effect was only shown with HMB for CWR, which means HMB was more efficient than AOC in MCP production and a part of HMB seemed to be efficiently utilized as a nitrogen source. This finding is consistent with the result of Jouany et al. (1998), who reported that adding AOC had no effect on in vitro ammonia-N. The result with HMB agreed with the report of Vázquez-Añón et al. (2001), who found that addition of HMB in the feed improved microbial protein synthesis and reduced rumen ammonia-N. However, both AOC and HMB reduced ammonia-N and enhanced MCP for CS. In addition, a HMB×AOC interaction in MCP for the two roughages was observed. The reason for a different effect of AOC on ammonia-N of CWR and CS is not clear. This phenomenon was also further enhanced by a larger HMB×AOC effect on CS ammonia-N. The reason for this difference may be due to the high nitrogen content of CS.
Volatile fatty acid
VFA is the product of microbial fermentation of carbohydrates in the rumen; therefore, increased ruminal VFA concentrations are often assumed to be a result of this fermentation. From our study, total VFA of CWR and CS was increased by supplementing AOC and HMB. Frumholtz et al. (1989) reported the addition of AOC resulted in an increase of total VFA. Martin et al. (2013) showed HMB and isopropyl ester of HMB (HMBi) supplementation increased VFA concentrations in the rumen. However, Whelan et al. (2013) showed supplementary HMBi had no effect on rumen pH, VFA or ammonia-N in lactating dairy cows offered a low crude protein diet. Though both HMB and AOC increased total VFA, their effects on molar proportions of each VFA were different with the two roughage sources. That different fermentation patterns were shown by the two forages was probably due to their different chemical compositions. As shown in Table 1, CS had more protein, less organic matter, NDF and ADF than CWR which led to its easily being digested by microbes in the rumen. Noftsger et al. (2003) showed supplementation of HMB did not affect total VFA but observed differences in concentration of individual VFA which was different from our findings. In addition, AOC showed better effect on propionate feasibly due to enhanced soluble carbohydrate digestion by its α-amylase. This finding was inconsistent with Carton et al. (1993), who concluded supplemental AOC did not affect total VFA and VFA proportions. There were HMB×AOC interactions in total VFA for CS. In combination, the two additives had significant effects on total VFA.
Rumen microbial population
In the current study, adding HMB and AOC had different effects on protozoa and fungi. Despite HMB supplementation significantly enhancing GP and total VFA of CWR, it did not increase the population of three cellulolytic species. An increase of F. succinogenes and R. albus was only observed with HMB for CS. Blake et al. (1986) suggested that HMB might stimulate a more metabolically active bacterial population. Martin et al. (2013) reported HMB supplementation had no effect on the total protozoa concentration, increased rumimal abundance of F. succinogenes and R. flavefaciens, but did not improve rumen fibrolytic activity. Supplementing AOC significantly increased the populations of fungi and F. succinogenes in the two roughage sources. In addition, AOC only significantly increased R. albus in CWR compared with CS because CWR had a greater amount of fiber substrate to act upon. It may be that these results are related to the NDF and/or starch proportion of the substrates. Although AOC does not produce the enzymatic machinery to completely depolymerize structural carbohydrates to simple sugars, it does produce enzymes that cause partial depolymerization (Boing, 1983) and it aids rumen cellulytic bacteria in completing the depolymerization of cellulosic material to simple sugars (Autrey et al., 1975). Newbold (1997) also presented data indicating that AOC stimulated the attachment of rumen microbes to plant fiber, which may explain how small quantities of fungi can have a significant effect on fiber degradation. In addition, there were interactions between HMB and AOC in fungi and R. flavefaciens of CWR and in R. albus of CS. The larger HMB×AOC effects on fungi and R. flavefaciens with AOC could partly be explained by Schmidt et al. (2004) who found that AOC accelerated the production and maturation of zoospores of Neocallimastix frontalis EB 188, along with an elevated production of protein and carboxymethy cellulase (CMCase). The mechanisms by which these supplements could impact microbial digestion in the rumen have not yet to be clarified. Nevertheless further research is warranted to understand their exact mode of action.
CONCLUSION
Results of this study suggest that addition of HMB and AOC had significant effect on GP, MCP, and total VFA of CWR and CS. However, as AOC affected the populations of fungi, F. succinogenes and R. flavefaciens in both roughages, HMB influenced F. succinogenes and R. albus only in CS. The HMB by AOC interactions were significant in MCP and total VFA which might be caused by collaboration of fungi, R. albus and R. flavefaciens.
ACKNOWLEDGMENTS
This work was supported by grants from National Key Basic Research Program of China (No. 2011CB100801), Novus International Inc. and the China Agriculture Research System (CARS-37).



