Effects of Adding Essential Oil to the Diet of Weaned Pigs on Performance, Nutrient Utilization, Immune Response and Intestinal Health
Article information
Abstract
The objective of this study was to evaluate the effects of adding essential oils to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. A total of 96 weaning pigs (8.37±1.58 kg) were allotted to one of three dietary treatments. The treatments consisted of an unsupplemented basal diet (negative control, NC) or similar diets supplemented with 0.01% of an essential oil product which contained 18% thymol and cinnamaldehyde (EOD) as well as a diet supplemented with 0.19% of an antibiotic mixture which provided 150 ppm chlortetracycline, 80 ppm colistin sulfate and 50 ppm kitasamycin (positive control, PC). Each treatment was provided to eight pens of pigs with four pigs per pen. Over the entire 35 d experiment, ADG and fecal score were improved (p<0.05) for pigs fed the PC and EOD compared with the NC. Dry matter and crude protein digestibility as well as lymphocyte proliferation for pigs fed the PC and EOD diets were increased significantly compared with NC (p<0.05). IGF-I levels in plasma were significantly increased (p<0.05) in pigs fed the PC diet compared with pigs fed the NC diet. Interleukin-6 concentration was lower (p<0.05) and the tumor necrosis factor-α level was higher (p<0.05) in the plasma of pigs fed the EOD diet than the NC diet. Plasma total antioxidant capacity level increased (p<0.05) in pigs fed the EOD diet compared with pigs fed the NC. Villus height to crypt depth ratio in the jejunum was greater (p<0.05) in pigs fed the PC and EOD diets than the NC. The numbers of E. coli in the cecum, colon and rectum were reduced (p<0.05) in pigs fed the PC and EOD diets compared with the control. In the colon, the ratio of Lactobacilli to E. coli was increased (p<0.05) in pigs fed the EOD diet compared with NC diet. Total aerobe numbers in the rectum were decreased (p<0.05) in pigs fed the PC and EOD diets compared with the control. Collectively, these results indicate that blends of essential oils could be a candidate for use as an alternative to traditional antibiotics in weaning pig diets.
INTRODUCTION
Antibiotics have been widely used in swine production, especially for starter pigs, because around weaning, pigs usually exhibit impaired growth, increased incidence of diarrhea and other diseases (Cromwell, 2002; Boudry et al., 2004), as well as undergo severe changes in the histomorphology of the intestine (Spreeuwenberg et al., 2001). However, increased resistance of bacteria is a potential problem caused by routine use of sub-therapeutic antibiotics (van den Bogaard and Stobberingh, 1999) and therefore alternatives are being sought (Kong et al., 2007a; Hao et al., 2012).
In recent years, there has been increased interest in the use of phytogenetic feed additives as a potential replacement for antibiotics (Windisch et al., 2008). Essential oils (EO) are volatile lipophilic materials derived from plants and there are more than one hundred kinds of essential oils (Wei and Shibamoto, 2007). Previous studies with pigs indicate that essential oils may improve pig performance (Cho et al., 2006; Kroismayr et al., 2008a), nutrient digestibility (Yan et al., 2010), immune status (Nofrarias et al., 2006) and the intestinal ecosystem (Manzanilla et al., 2009).
The effects of essential oils in pigs have been variable because of different study conditions (Janczyk et al., 2009), differences in the type of essential oils used as well as variation in the dose provided (Windisch et al., 2008). The present study was conducted to determine the effects of dietary supplementation with an essential oil blend on the performance, nutrient digestibility, immune status, intestinal morphology, and intestinal microbiology of weaned pigs compared with an antibiotic growth promoter.
MATERIALS AND METHODS
Essential oil products
The commercial EO product was supplied by Danisco Animal Nutrition in this study. The blend contained 18% thymol and cinnamaldehyde (EOD).
Performance
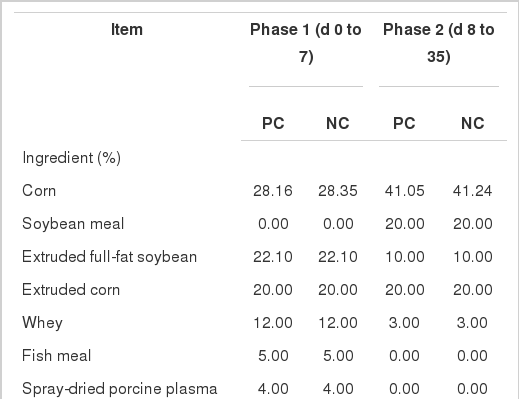
All the procedures of this study were approved by the China Agricultural University Animal Care and Use Committee (Beijing, China). A total of 96 crossbred (Duroc ×Landrace×Yorkshire) piglets, weighing an average of 8.37±1.58 kg, were used in this study. Piglets in each litter were kept with their sow, and there’s no creep feed when lactating. Piglets were allotted to one of three dietary treatments on the basis of weight and gender in a randomized complete block design (Table 1).
The treatments consisted of an unsupplemented basal diet (negative control, NC) or similar diets supplemented with 0.01% of an essential oil product which contained 18% thymol and cinnamaldehyde (EOD) as well as a diet supplemented with 0.19% of an antibiotic mixture which provided 150 ppm chlortetracycline, 80 ppm colistin sulfate and 50 ppm kitasamycin (positive control, PC). Diets were divided into two phases (d 0 to 7 and d 8 to 35). Chromic oxide (0.25%) was added to all 2nd phase diets as a digestibility marker. All diets were fed in mash form.
The experimental pigs were housed in 2×3 m raised pens equipped with a mesh floor. The temperature of pig barns was controlled at 25°C. The light schedule was 12 h light: 12 h dark. All pigs had free access to feed and water throughout the 5-wk feeding trial. Each treatment was fed to eight pens of pigs with each pen containing four pigs (two male and two female piglets).
Measurements
Pigs were weighed individually on the morning of d 0, 7 and 35 of the experiment. Feeders were also weighed at these times in order to monitor feed consumption. These values were then used to calculate the weight gain, feed intake and feed conversion ratio for each pen.
Fecal consistency was visually assessed each morning of the experiment by observers unaware of the treatments using a modification of the method described by Liu et al. (2010). Fresh excreta were ranked using the following scale: 1 = solid; 2 = semi-solid; 3 = semi-liquid; and 4 = liquid.
From d 33 to 35 of the experiment, approximately 50 g of feces was collected daily from each pen and the samples were stored at −20°C. The three day collection of feces was thawed, pooled by pen and then oven dried at 60°C for 72 h. All samples were ground to pass through a 1.0 mm screen (40 mesh) before analysis. Nutrient digestibility was determined using the indicator method. The equation used was as follows:
Where, ND is the apparent total tract digestibility, DC is the content of Cr2O3 in the assay diet (%), FN is the content of a nutrient in the feces (%), FC is the content of Cr2O3 in the feces (%), DN is the content of a nutrient in the assay diet (%).
Two pigs were randomly selected from each pen and 5 ml of blood was obtained by vena cava puncture from each pig using a heparinized vacutainer tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA) on the morning of d 35 after an overnight fast. Half of the each blood sample was centrifuged at 3,500 rpm (Heraeus Biofuge 22R Centrifuge, Hanau, Germany) for five min and the plasma obtained and immediately stored at −20°C for analysis. The other half of the blood samples was used immediately for assessment of the Lymphocyte Proliferation Index.
At the end of the 5-wk study, 15 pigs (five pens from each treatment and one pig per pen) chosen from the pigs which provided blood samples were humanely killed by exsanguination after electrical stunning to obtain intestinal tissues and digesta samples as described previously (Shen et al., 2009). Intestinal tissue from the middle of the duodenum, jejunum, and ileum were aseptically isolated, flushed with 0.9% salt solution, fixed in 10% formaldehyde-phosphate buffer, and kept at 4°C for microscopic assessment of mucosa morphology. The digesta in the cecum, colon, and rectum were collected and immediately immersed in liquid nitrogen and then stored at −80°C for subsequent microbial counting.
Chemical analysis of feed and feces
At the beginning of the experiment, feed samples were collected and ground to pass through a 1.0 mm screen (40 mesh). Analysis for dietary and fecal dry matter, crude protein, calcium, and total phosphorus were conducted according to the methods of AOAC (1990). Gross energy was measured by an automatic adiabatic oxygen bomb calorimeter (Parr 6300 Calorimeter; Moline, IL, USA). Chromium concentration was determined by an atomic absorption spectrophotometer (Hitachi Z-2000 Automatic Absorption; Spectrophotometer, Tokyo, Japan) according to the method of Williams et al. (1962).
The amino acid concentration of the diets was analyzed after the diets were ground through a 60 mesh screen. Feed samples were hydrolyzed in 6 N HCl (10 ml) at 110°C for 24 h under nitrogen atmosphere. Sulphur containing amino acids were measured after performic acid oxidation (AOAC, 1990). Trytophan content was determined colorimetrically after alkaline hydrolysis following the procedures described by Miller (1967).
Measurement of immunity, cytokines, and antioxidant indices in plasma
Plasma concentrations of immunoglobulins (IgA, IgG and IgM), interleukin-1, interleukin-6, tumor necrosis factor-α and insulin-like growth factor-I were quantified using an enzyme-linked immunosorbent assay specific for swine (R & D, Minneapolis, MN, USA), according to the manufacturer’s instructions. Assays were counted in duplicate using a TEACAN Plate Reader (TEACAN Asia, Shanghai, China). Plasma albumin level was measured with an Automatic Biochemical Analyzer (RA-1000; Bayer Corp., Tarrytown, NY, USA) using colorimetric methods and the instructions from the manufacturer’s corresponding reagent kit (Zhongsheng Biochemical Company, Beijing, China). Assay of plasma total antioxidant capacity, superoxide dismutase, glutathione peroxidase and malondialdehyde levels were conducted by spectrophotometric methods using a spectrophotometer (Leng Guang SFZ1606017568, Shanghai, China) following the instructions of the kit’s manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Lymphocyte proliferation assay
Lymphocyte proliferation was conducted using a colorimetric assay, with 3-(4, 5-dimethlthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, M-2128, Sigma, St Louis, MO, USA) in cultures of purified peripheral blood mononuclear cells as described previously (Liu et al., 2003; Li et al., 2006). Blood was centrifuged at 3,000×g for 10 min at room temperature in 5 ml of histopaque (density 1.077; Sigma-Aldrich, Dorset, UK) in 10-ml centrifuge tubes. The separated cells (approximately 1 ml) were transferred into another 10-ml centrifuge tube containing 4 ml of Roswell Park Memorial Institute Medium 1640 (RPMI; Gibco Life Technologies, New York, NY, USA) and mixed thoroughly.
After centrifugation at 2,000×g for 10 min at room temperature, the supernatant was discarded and the pellet was re-suspended in 4 ml of RPMI. After repeating this process 2 additional times, the pellet was re-suspended in 4 ml of RPMI supplemented with penicillin G (100 U/ml; Merck KGaA, Darmstadt, Germany), streptomycin (100 μg/ml; Sigma St Louis, MO, USA), and 10% heat-inactivated newborn calf serum (HyClone Laboratories Inc., Logan, UT, USA), counted, and plated into polypropylene 96-well culture dishes (Costar Corp., Cambridge, MA) at 2 ×106 cells/ml.
Lymphocyte mitogen concanavalin A (Type IV, C-2010, Sigma St Louis, MO, USA) was added at a final concentration of 16 mg/ml of culture medium, after which the plates were incubated at 37°C in an incubator in an atmosphere of 5% CO2 (Heraeus, Hongkong, China) for 66 h Subsequently, 10 μl of MTT solution (5 mg of MTT/ml in phosphate-buffered saline (0.07 M, pH 7.6)) was added to each well, and the plates were incubated at 37°C for 6 h. After incubation, 100 μl of 10% sodium dodecyl sulfate (Sigma St Louis, MO, USA) in 0.04 M HCl was added to lyse the cells and solubilize the MTT crystals. The plates were read at 570 nm using an automated microplate reader (Model 550, BioRAD Laboratories Inc., Hercules, CA). Lymphocyte proliferation was expressed as a proliferation index, which was calculated as the absorbance of wells incubated with concanavalin A divided by the absorbance of wells incubated without concanavalin A.
Investigation of mucosa morphology
The fixed samples from the intestine were embedded in paraffin wax, then, cut at a thickness of 5 μm and stained with hematoxylin and eosin as described by Shen et al. (2009). Villus height and crypt depth of the intestines were examined with a microscope (Olympus CK 40, Olympus Optical Company, Shenzhen, China) at 40×magnification using samples with more than 10 well oriented intact villi (3 replicates).
Microbiological analyses
Bacterial counts in the digesta obtained from the large intestine were measured by the plate-count technique (Wang et al., 2011). One gram of digesta was homogenized in salt solution (0.9% NaCl) and serially diluted from 10−1 to 10−9 for analysis of E. coli (MacConkey agar, Beijing Haidian Microbiological Culture Factory, Beijing, China), Lactobacilli (MRS agar, De Man, Rogosa, Sharpe, Oxoid Ltd., CM0361. anaerobic chamber), total anaerobes (plate-count agar, anaerobic chamber), and total aerobes (plate-count agar). All plates were incubated for 48 h in 37°C. Numbers of bacteria were expressed as log10 CFU per gram.
Statistical analysis
Data were analyzed as a completely randomized design using the GLM procedure of SAS (SAS Inst. Inc., Cary, NC). The pen was considered the experimental unit with initial body weight used as a covariate. Lsmeans in the GLM procedure were used for multi-comparison of corrected mean performance. The alpha level used in the determination of significance for all the analysis was p<0.05 with trends indicated at p<0.10.
RESULTS
Performance and fecal consistency
The effects of dietary essential oils and antibiotics on performance and fecal consistency data are presented in Table 2. During phase 1, from d 0 to 7, there were no significant differences among the treatments. From d 8 to d 35, and during the overall experiment, pigs fed the PC and EOD diets had increased weight gain (p<0.05) and improved fecal scores (p<0.05) compared with pigs fed the NC. Feed intake and feed conversion ratio did not differ among treatments.
Apparent digestibility
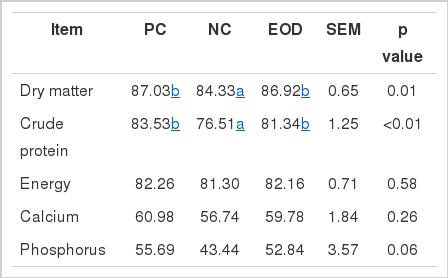
The effects of dietary essential oils and antibiotics on total tract apparent nutrient digestibility are shown in Table 3. The dry matter (p<0.05) and crude protein (p<0.05) digestibility for pigs fed the PC and EOD diets was significantly higher than for pigs fed the NC. In addition, the digestibility of phosphorus tended to be higher (p<0.10) for pigs fed the PC and EOD diets than for pigs fed the NC.
Plasma assay for IGF-I, immune status, cytokines and antioxidative capacity
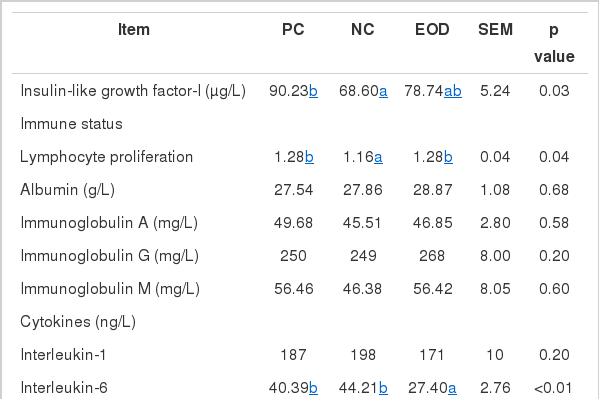
Table 4 shows the effect of dietary essential oil and antibiotics on IGF-I, immune status, cytokines and antioxidative status in the plasma of weaned pigs. IGF-I levels in plasma were significantly increased (p<0.05) in pigs fed the PC than for pigs fed the NC. Lymphocyte proliferation increased (p<0.05) in pigs fed the PC and EOD diets compared with the NC diet. There were no differences in other immune status parameters between treatments. Interleukin-6 concentration was lower (p<0.05) and tumor necrosis factor-α was higher (p<0.05) in the plasma of pigs fed the EOD diet than for pigs fed the NC. There’s no significantly difference between PC and NC treatment. Total antioxidant capacity also increased (p<0.05) in pigs fed the EOD diet compared with the NC.
Small intestinal morphology measurements
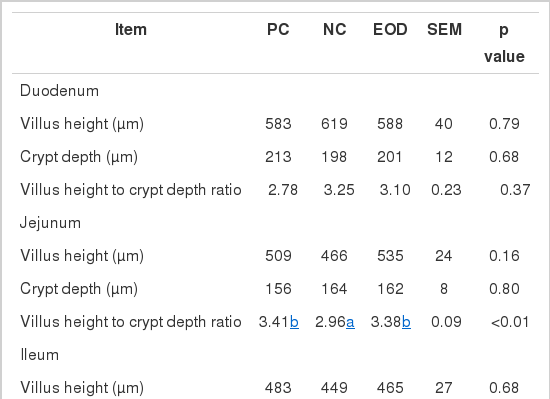
No dietary effects were found in villus height, crypt depth and the villus height to crypt depth ratio in the duodenum and ileum as a result of dietary inclusion of antibiotics or essential oils (Table 5). However, villus height to crypt depth ratio in the jejunum was higher (p<0.05) in pigs fed the PC and EOD diets compared with the NC diet.
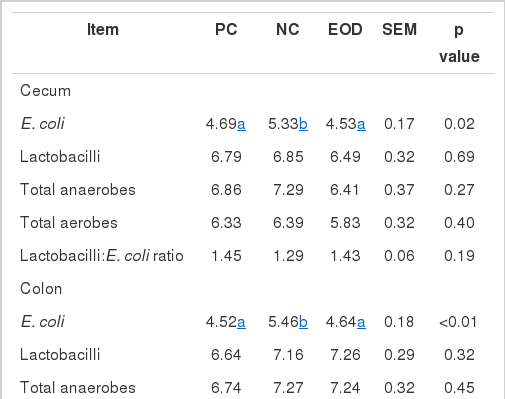
Composition of bacteria in the large intestine
The number of E. coli in the cecum, colon and rectum was reduced (p<0.05) in pigs fed the PC and EOD diets compared with the NC diet (Table 6). In the colon, the ratio of lactobacilli and E. coli was increased (p<0.05) in pigs fed the EOD diet compared with the NC diet, while there was no significant difference between PC and NC treatments. Total aerobe numbers in the rectum were decreased (p<0.05) in pigs fed the PC and EOD diets compared with the NC diet.
DISCUSSION
Diarrhea, low feed intake and nutrient digestibility were always the challenges for weaning pigs, present results showed that EOD could help young pigs face these problems. Diarrhea is a severe problem which is related to a higher mortality rate in newly weaned pigs (Osek, 1999). Diets lacking antibiotics could lead to an increased occurrence of diarrhea in weaning pigs (Fairbrother et al., 2005), an observation consistent with the current study. Dietary essential oils improved the fecal consistency compared with the negative control. Similarly, Manzanilla et al. (2004) showed positive effects on weaning diarrhea. Franca et al. (2008) reported that Ocimum selloi essential oil had antidiarrheal capability in mice. Many studies reported that phytogenic feed additives could enhance feed intake due to improvements in the flavor and palatability of feed (Windisch et al., 2008). However, other studies showed essential oils resulted in reduced feed intake (Jugl-Chizzola et al., 2006; Schone et al., 2006). In our experiment, feed intake was not influenced by EOD. The results of present study are similar to previous observations showing that weight gain was significantly increased by essential oil compared with control diets (Cho et al., 2006). Lien et al. (2007) found increased weight gain and improved feed efficiency by feeding phytogenic compounds. Other studies found there was a numeric improvement in weight gain and feed intake, but the difference was not significant (Kroismayr et al., 2008a b) and these results were also shown in the studies of Manzanilla et al. (2006) and Nofrarias et al. (2006).
The reason for increased growth in pigs fed the EOD diet compared with the NC may be partly due to the improvement in nutrient digestibility observed in our study, which is consistent with the study in grower-finisher pigs conducted by Yan et al. (2010). Amerah et al. (2011) reported a similar response in broilers, where a blend of essential oils (thymol and cinnamaldehyde) resulted in improved nitrogen digestibility. Similarly, Zitterl-Eglseer et al. (2008) reported a significant improvement of dry matter and crude protein digestibility by dietary supplementation of carvacrol and thymol essential oils. The change of morphology in the intestine would be followed by a change in nutrient digestion and absorption (Pluske et al., 1997), as an enhanced villus to crypt ratio has been shown to improve nutrient digestibility (Shen et al., 2009). In the current study, nutrient digestibility was improved significantly. We also observed villus height to crypt depth ratio in the jejunum was increased compared with the negative control. However, a study conducted by Kroismayr et al. (2008b) showed a trend of reduced villi length in jejunum and ileum by essential oils, where other previous reports showed this to be unchanged in pigs (Namkung et al., 2004; Nofrarias et al., 2006).
Plasma IGF-I levels often reflect nutritional status (Ketelslegers et al., 1995). There can be a positive correlation between plasma IGF-I and growth in pigs (Owens et al., 1999). In the current experiment, a significant enhancement of plasma IGF-I in pigs fed the PC diet could explain the increased weight gain compared with pigs fed the NC diet.
There was no change in immune parameters except for the increase in lymphocyte proliferation. However, other studies have found no effects of essential oils or plant extracts on immune response (Manzanilla et al., 2004; Namkung et al., 2004). Interleukin-1, interleukin-6, and tumor necrosis factor-a are three major cytokines, which play an important role in cellular immunity (Johnson, 1997). High levels of cytokines like interleukin-6, could induce tissue damage (Pie et al., 2004), reduce the secretion of growth hormone (Johnson, 1997), and change the metabolism of energy and protein to negatively affect the performance of the animal (Spurlock, 1997). In the present experiment, we found a decrease of interleukin-6 in pigs fed the EOD compared with pigs fed the NC diet which is consistent with an improvement in performance, as shown in Mao et al. (2005), who found a decreased plasma interleukin-1β and increase in lymphocyte proliferation (ConA) by dietary Astragalus membranaceus glucan, while the current study only found a trend for an increase in conconavalin A induced lymphocyte proliferation. Our results were consist with previous studies which found herbal extract could improve the immune response in weaned pigs (Deng et al., 2007a; Kong et al., 2007b; Yin et al., 2008).
Most essential oils are well known for their antioxidation ability (Grassmann et al., 2001; Wei and Shibamoto, 2007). Plasma total antioxidant capacity is a marker of a non-enzymatic antioxidant defense system (Wang et al., 2008). Total antioxidant capacity was increased by dietary essential oils in the present study which indicates that essential oils may play a role in preventing endogenous lipids from peroxidation.
It has been reported that most diarrhea of pigs is caused by viral or enterotoxigenic E. coli (Fairbrother et al., 2005). One of the possible reasons for the reduced fecal consistency may the decreased number of intestinal E. coli observed in present study. As has been consistently shown in previous studies, essential oils could improve the ecology of the intestine by increasing the lactobacilli to enterobacteria ratio (Manzanilla et al., 2004), reducing ileal anaerobic and aerobic bacteria (Kroismayr et al., 2008a), and decreasing ceacal total bacteria (Castillo et al., 2006). Similar to in vitro experiments, essential oils had good antibacterial function (Burt, 2004). Also, Deng et al. (2007b) reported that polysaccharides from Cassiae Seeds could improve the intestinal microflora.
In conclusion, the results of present study indicate that dietary essential oils (thymol and cinnamaldehyde) improved performance and reduced the diarrhea probably by improving immune status, intestine ecology, and nutrient digestibility.
ACKNOWLEDGEMENTS
The authors sincerely acknowledge the financial support received from the Danisco Co. Ltd., NSFC (31072040) and SKLAN (2004DA125184F1211) of China. Thank Novus International for supplying the MHA.