Effects of Temperature during Moist Heat Treatment on Ruminal Degradability and Intestinal Digestibility of Protein and Amino Acids in Hempseed Cake
Article information
Abstract
The objective of this study was to evaluate ruminal degradability and intestinal digestibility of crude protein (CP) and amino acids (AA) in hempseed cake (HC) that were moist heat treated at different temperatures. Samples of cold-pressed HC were autoclaved for 30 min at 110, 120 or 130°C, and a sample of untreated HC was used as the control. Ruminal degradability of CP was estimated, using the in situ Dacron bag technique; intestinal CP digestibility was estimated for the 16 h in situ residue using a three-step in vitro procedure. AA content was determined for the HC samples (heat treated and untreated) of the intact feed, the 16 h in situ residue and the residue after the three-step procedure. There was a linear increase in RUP (p = 0.001) and intestinal digestibility of RUP (p = 0.003) with increasing temperature during heat treatment. The 130°C treatment increased RUP from 259 to 629 g/kg CP, while intestinal digestibility increased from 176 to 730 g/kg RUP, compared to the control. Hence, the intestinal available dietary CP increased more than eight times. Increasing temperatures during heat treatment resulted in linear decreases in ruminal degradability of total AA (p = 0.006) and individual AA (p<0.05) and an increase in intestinal digestibility that could be explained both by a linear and a quadratic model for total AA and most individual AA (p<0.05). The 130°C treatment decreased ruminal degradability of total AA from 837 to 471 g/kg, while intestinal digestibility increased from 267 to 813 g/kg of rumen undegradable AA, compared with the control. There were differences between ruminal AA degradability and between intestinal AA digestibility within all individual HC treatments (p<0.001). It is concluded that moist heat treatment at 130°C did not overprotect the CP of HC and could be used to shift the site of CP and AA digestion from the rumen to the small intestine. This may increase the value of HC as a protein supplement for ruminants.
INTRODUCTION
Proteins fed to ruminants are broken down by microbes in the rumen and used for microbial protein synthesis. Although some feed proteins escape the rumen without being degraded. Amino acids (AA) that are required by the ruminant originate from both microbial protein and rumen undegradable protein (RUP) and, to a lesser extent, from endogenous protein (NRC, 2001). Strategies that are used to increase conversion of feed N into meat or milk include feeding to achieve greater microbial protein synthesis, to balance the supply of rumen degradable protein (RDP) and RUP, and to improve the supply of essential AA (Schwab et al., 2005). Accurate determination of the amount of RDP and the intestinal digestibility of RUP is required to estimate feed protein available to ruminants by nutritional models. Individual AA have been shown to differ with respect to their rumen degradability (Weisbjerg et al., 1996; Harstad and Prestløkken, 2001), so the RUP AA profile in a feed may differ from the feed AA profile. Hence, it may be incorrect to use the AA composition of the feed when predicting the supply of individual dietary AA to the small intestine.
A range of feed processing methods are applied to protect supplemented feed protein from microbial degradation in the rumen and thereby increase the amount of AA available for digestion in the small intestine. Heat treatment is a common method that can be performed in a number of ways, including moist heat treatment of feed, using an autoclave with a positive relationship between steam pressure and temperature (Van der Poel et al., 2005). Several in situ and in vitro studies demonstrated that it is possible to shift protein digestion in different oilseed feedstuffs from the rumen to the small intestine by heat treatment, without decreasing total digestibility (McKinnon et al., 1995; Dakowski et al., 1996; Mustafa et al., 1999a). However, temperatures that are too high may overprotect the protein, resulting in a decrease in post-ruminal availability (McKinnon et al., 1995; Dakowski et al., 1996).
In recent years, there has been increasing interest in using alternative protein crops in livestock production. Hemp (Cannabis sativa L.) is an ancient crop, cultivated for fiber and oil, that has received renewed attention during the last decade. The residue left after mechanical or solvent extraction of the oil from hempseed is a cake or a meal rich in protein and fiber (Callaway, 2004). A few studies have been published investigating the use of hempseed as a protein feed. These studies included hempseed in diets for lambs (Mustafa et al., 1999b ; Karlsson and Martinsson, 2011), growing cattle (Gibb et al., 2005; Hessle et al., 2008; Turner et al., 2008) and dairy cows (Karlsson et al., 2010). Mustafa et al. (1999b) found a low effective protein degradation (EPD) of 394 g/kg CP in hempseed meal estimated in situ. An in vitro study by Karlsson et al. (2009) showed that cold pressed hempseed cake (HC) had an EPD value of 330 g/kg CP. Contrary to these observations, the estimated EPD value of HC was 709 g/kg CP in an in situ study (Karlsson and Martinsson, 2011). In addition, a low intestinal RUP digestibility was found, resulting in a much lower value for intestinally available CP of HC (90 g/kg CP) than the value for hempseed meal (654 g/kg CP) reported by Mustafa et al. (1999b). Heat treatment may be an option to decrease EPD in HC and thereby increase RUP and supply of AA to the post-ruminal tract. A high intestinal digestibility of RUP is required to ensure that there is no decrease in total available CP from feed. To the authors’ knowledge, there are no published studies investigating possibilities for altering site of protein digestion of HC. The objective of this study was to evaluate ruminal degradability and intestinal digestibility of CP and AA in HC, exposed to moist heat treatment at various temperatures.
MATERIALS AND METHODS
Feed samples and chemical analysis
Hempseed (Cannabis sativa L., cv. Finola) was cold-pressed to produce HC (Täbypress Type 90, Skepsta Maskiner AB, Örebro, Sweden) by a commercial oil producer. The HC was ground and passed through a 2 mm screen (Thomas - Willey laboratory mill Model 4, PA, USA) and samples of approximately 100 g were autoclaved for 30 min at 110°C (HC110), 120°C (HC120) or 130°C (HC130), using a direct steam heated sterilizer (Consolidated Stills and Sterilizers, Boston, MA, USA). Different temperatures were chosen in order to allow us to evaluate the effects of increasing temperature on rumen degradability and intestinal digestibility of protein and AA in HC. After an exhaust period of 1 min in the autoclave, samples were allowed to cool for 24 h before being sealed in plastic bags. A sample of untreated HC was kept as a control (HC0).
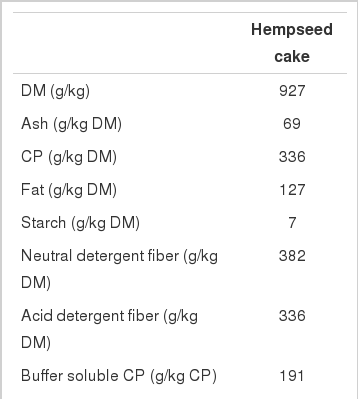
Chemical composition data presented in Table 1 were determined as described by Karlsson and Martinsson (2011). Otherwise, dry matter (DM) content was determined by drying at 100°C for 24 h, and CP content was determined according to AOAC (1984) as Kjeldahl-N×6.25 using a 2020 Digestor and a 2300 Kjeltec Analyser Unit (Foss Tecator AB, Höganäs, Sweden).
In situ incubations
Rumen degradability of HC protein was determined by measurements of in situ CP disappearance (Ørskov and McDonald, 1979). Approximately 0.5 g of feed was weighed into Dacron bags (Ankom R510, 50×100 mm, 50 (μm) and incubated in duplicates in the rumen of a cannulated lactating cow (Montbeliarde×(Jersey×Holstein)) for 2, 8, 16, 24 or 48 h. The cow was fed approximately 3% of body weight of a diet based on corn silage (33%), ground corn (20%), alfalfa hay (16%), cottonseed (7.5%), soybean meal (7.4%), soybean hulls (3.7%), canola meal (3.7%), corn distiller grain (2.8%) and molasses (2.6%) on DM basis. Bags were soaked in 39°C tap water for 15 min, prior to incubation in the rumen. After removal from the rumen, bags were rinsed in cold tap water until the runoff was clear, and oven-dried at 55°C for 48 h. Zero-time disappearance, assumed to be the soluble fraction, was determined by soaking duplicates of each treatment in 39°C tap water for 15 min (rinsing every third min for 1 min). All residues were analyzed for CP as described above. Proportions of RDP and RUP were calculated according to the equation of Ørskov and McDonald (1979) assuming a passage rate of 0.06/h.
To obtain rumen undegradable material, 12 additional bags per treatment, each containing a 1.2 g sample, were incubated in the rumen for 16 h (representing the feed that would escape from the rumen at a passage rate of 0.06/h). Samples were washed and dried as described above and then pooled before CP determination and estimation of intestinal digestibility. The in situ procedure described above was repeated in two consecutive runs using the same feeds and the same cow.
In vitro intestinal digestibility
Intestinal digestion of the RUP fraction was estimated using the three-step in vitro procedure, TSP (Calsamiglia and Stern, 1995), modified by Gargallo et al. (2006). Approximately 1.0 g of the pooled rumen-exposed residue was weighed into Dacron bags (Ankom R510, 50×100 mm, 50 (μm) (four per treatment) and placed in a DaisyII incubator (ANKOM, Fairport, NY). Samples were incubated in 2 L of pre-warmed 0.1 N HCl solution adjusted to pH 1.9 and containing 1 g/L of pepsin (Sigma P-7000, Sigma Chemicals Co., St Louis, MO, USA), they were rotated constantly at 39°C for 1 h. Samples were rinsed in cold tap water until the runoff was clear before they were incubated in 2 L of pre-warmed pancreatin (Sigma P-7545, Sigma Chemicals Co., St Louis, MO, USA) solution (0.5 M KH2PO4 buffer standardized at pH 7.8 and containing 50 ppm of thymol and 3 g/L of pancreatin), rotated constantly at 39°C for 24 h. After incubation, samples were rinsed in cold tap water until the runoff was clear and oven-dried at 55°C for 48 h. Residues were pooled by sample for CP and AA analyses.
Amino acid analysis
Samples of HC (HC0, HC110, HC120 and HC130) were analyzed for AA by a commercial laboratory, using a Hitachi High-Technologies Model L-8900 AA analyzer. The instrument incorporated a cation exchange column, multiple sequential lithium-based eluents, and lithium hydroxide for column regeneration. Absorbance was measured at 440 and 570 nm following post-column color development by Ninhydrin reagent at 131°C. Degradability and digestibility of all individual AA were calculated on the basis of quantification by acid-stable hydrolysis, performed on the intact HC, after 16 h in situ incubation and after the TSP from the two repeated runs. The cystine and methionine content in the intact HC from the second run were also quantified by means of performic acid hydrolyzed AA analysis.
Because the 16 h residue may not represent the protein that would escape from the rumen at a passage rate of 0.06/h, ruminal AA degradability was calculated using the computed RUP value. Assuming that the AA profile of 16 h CP in situ residue will be representative for the RUP, rumen degradability and intestinal digestibility of AA (g/kg AA) were calculated using the equations:
Statistical analyses
Data were examined by analysis of variance, using the MIXED procedure in SAS (Littell et al., 2006). The content, ruminal degradability and intestinal digestibility of CP and AA were evaluated using the model:
where: μ is the overall mean, αi is the main effect of treatment (i = 4; HC0, HC110, HC120, HC130), bj is the random effect of run (j = 2) and eij is the residual error. Treatment effect was divided into the following orthogonal comparisons: i) effect of heat treatment (control vs other), ii) linear and iii) quadratic effects of temperature during heat treatment. Differences in AA degradability, as well as AA digestibility, within each treatment were evaluated using the model presented above, where: μ is the overall mean, αi is the main effect of individual and total AA (i = 18), bj is the random effect of run (j = 2) and eij is the residual error. Tukey’s adjustment was used for multiple comparisons of individual and total AA. In all statistical analyses, differences were considered significant at p<0.05. The effect of treatment on cystine and methionine content in the intact HC could not be analyzed statistically because there was no replication for the alternative hydrolysis method used for quantification.
RESULTS
Ruminal degradation and intestinal digestion of crude protein
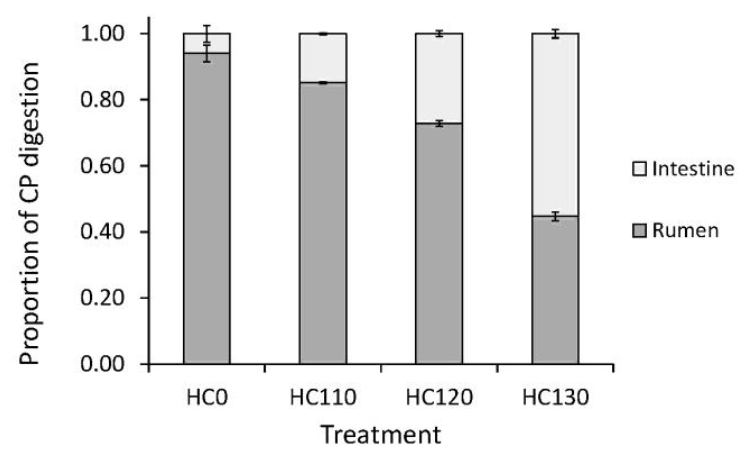
In situ ruminal CP disappearance, for the heat treated HC and untreated control, is shown in Figure 1. Heat treatment decreased CP solubility (p<0.001) and rate of in situ CP disappearance (p = 0.033), while it increased RUP (p = 0.002), intestinal digestibility (p = 0.002) and intestinal available dietary CP (p<0.001), compared with the control (Table 2). The amount of indigestible CP was not different in the HC0 and heat treated HC samples (p = 0.133). There was a linear increase in RUP (p = 0.001) and intestinal digestibility of RUP (p = 0.003) as temperature increased during heat treatment. Consequently, intestinal available dietary CP increased by a factor of more than eight as a result of the 130°C treatment (Table 2). Changes in site of digestion resulting from moist heat treatment of the HC, are shown in Figure 2 as proportions of feed CP degraded in the rumen and digested in the small intestine.

Proportion of in situ crude protein (CP) disappearance in hempseed cake moist heat treated for 30 min at 110, 120 or 130°C and in the untreated control (HC0).

In situ crude protein (CP) disappearance and in vitro CP digestion (g/kg CP, if not otherwise stated) of hempseed cake, untreated (HC0) or moist heat treated at 110, 120 or 130°C
Ruminal degradation and intestinal digestion of amino acids
There were no differences in individual (p>0.1) or total AA (p = 0.741) content between HC0 and heat treaded HC samples (Table 3). The most abundant AA in HC was glutamic acid, accounting for approximately 181 g/kg of total AA. Heat treatment decreased ruminal degradability of total AA and individual AA (p<0.05), except methionine, and there were linear decreases (p<0.05) with increasing temperature during heat treatment (Table 4). Intestinal digestibility of total AA and individual AA, except cystine and methionine, was affected by heat treatment. Higher temperatures resulted in increased intestinal digestibility that could be explained by either a linear or a quadratic model for most of the AA (p<0.05) (Table 5).
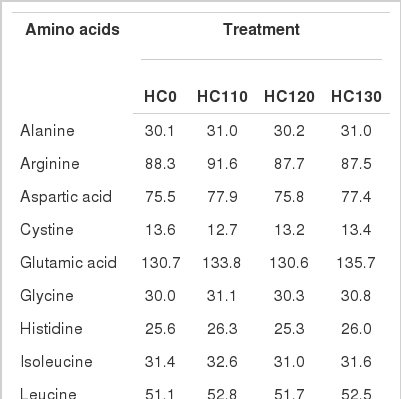
Amino acid composition (g/kg crude protein) of hempseed cake, untreated (HC0) or moist heat treated at 110, 120 or 130°C

Ruminal degradability of amino acids (g/kg amino acid) in hempseed cake, untreated (HC0) or moist heat treated at 110, 120 or 130°C

In vitro intestinal digestibility of rumen undegraded amino acids (g/kg amino acid) in hempseed cake, untreated or moist heat treated at 110, 120 or 130°C
There were differences between rumen AA degradabilities and between intestinal AA digestibilities within treatments (p<0.001). Compared to total AA, cystine and methionine had higher ruminal degradability within all treatments. Arginine and phenylalanine had higher and lower degradability, respectively compared with total AA degradability in HC0 and HC110. Intestinal digestibility of AA differed from total AA digestibility, particularly in HC130 where arginine, cystine and methionine had higher digestibility (p<0.01) and phenylalanine and proline had lower digestibility (p<0.01) compared with that of total AA.
DISCUSSION
Amino acid content
The most abundant AA found in HC (glutamic acid, arginine and aspartic acid) were the same as those found in earlier studies of hempseed protein (Callaway, 2004; Wang et al., 2008). The amounts of total AA in the CP of HC were similar to rapeseed meal heated at 150°C, but lower than untreated rapeseed meal (Dakowski et al., 1996). Total AA content ranged from 784 to 725 g/kg CP in rapeseed meal, untreated or heat treated at 130, 140 and 150°C. They found that higher temperatures resulted in lower contents of total AA. This effect on total AA was not found in the current study, however we did not evaluate temperatures higher than 130°C.
For milk production, methionine and lysine are often considered to be the most limiting AA (NRC, 2001); histidine has been found to be the most limiting AA in grass silage-based diets (Vanhatalo et al., 1999). Therefore, the content of these AA in a protein supplement could be of special interest. Metheonine in HC was higher, while lysine and histidine in HC were lower, compared with findings of Wang et al. (2008) for hempseed protein isolate. Wang et al. (2008) found hempseed protein to be a richer source of methionine (13.9 g/kg) than soya protein. However, the HCl-hydrolysis method that was used in their study could have resulted in an underestimate of the methionine content of both protein sources. Wang et al. (2008) reperted a lysine content of 41.6 g/kg and a histidine content of 28.1 g/kg for hempseed protein isolate. One explanation to the lower values found in the present study could be that the CP fraction contains some NPN, which causes a dilution of the AA content in this fraction compared with isolated protein.
Ruminal degradation
Effects of heat treatment on rumen CP degradability that were observed in this study are consistent with findings of several studies where oilseed protein feeds have been evaluated. For instance, Dakowski et al. (1996) observed a decrease in EPD of rapeseed meal measured in situ from 730 g/kg in untreated meal to 150 g/kg in meal heated to 150°C. Mustafa et al. (1999a) increased RUP after 12 h in situ incubation from 120 to 615 g/kg CP, and decreased in vitro EPD from 747 to 445 g/kg CP, by autoclaving mustard meal at 127°C. There are several factors that may influence the effect of heat treatment, these include temperature, time, moisture level and presence of sugars (Wallace and Falconer, 1992; Van der Poel et al., 2005). Autoclaving sunflower seeds for 10, 20 or 30 min increased RUP by 139, 143 and 164%, respectively, compared with the untreated control (Mustafa et al., 2003). Although there was little further reduction in CP degradability as heating time increased, the amount of RUP was higher when heated for 30 min. In the present study, we chose to vary temperature but maintain the same heating time, because temperature per se has been shown to have a clearer effect on in situ CP disappearance than the duration of heating (McKinnon et al., 1995).
Decreases in ruminal CP degradability with increased temperatures can be explained by a decrease in the soluble fraction (wash-out fraction) as well as the tendency towards a decreasing estimated rate of degradation that was found in the present study (Table 2). These results are in agreement with findings reported by Mustafa et al. (1999a), who fractionated true protein according to Sniffen et al. (1992) and found that autoclaving mustard meal resulted in significant increases in the intermediate and slowly degradable fractions (B2 and B3), while there was a decrease in the rapidly degradable fraction (B1).
Even though several studies have shown that heat treatment is an effective method to decrease degradation of CP in the rumen, studies evaluating production responses of feeding protected protein feeds have produced differing results. Santos et al. (1998) reviewed the literature and concluded that increasing the amount of RUP in the diets of dairy cows did not consistently improve milk production. Increases in RUP often result in decreased RDP and changes in absorbed AA profiles. Jones et al. (2001) found that only primiparous cows increased their milk yield when fed heated rather than unheated canola. In a meta-analysis by Huhtanen and Hristov (2009), ruminal CP degradability did not appear to be a significant factor in predicting milk protein yield or efficiency of N utilization for milk protein synthesis. Hence, in vivo measurements are needed to determine whether heat treatment of HC is genuinely beneficial to animal performance.
Ruminal degradability of CP and total AA determined in situ have been shown to be similar, but differences have been found between feedstuffs and between individual AA (Weisbjerg et al., 1996; Harstad and Prestløkken, 2001). In the present study, cystine and methionine had a higher ruminal degradability relative to total AA for all HC treatments. Hence, their degradability would be underestimated using RDP or total AA degradability as a predictor, especially for the highest temperature treatment. However, degradability and digestibility of AA were calculated using values determined by acid-stable hydrolysis. This procedure typically results in yields below the correct values for the sulfur-containing AA cystine and methionine. These two AA should be quantified by performic acid hydrolyzed amino acid analysis to achieve more reliable values for ruminal degradation and intestinal digestion.
Intestinal digestion
The purpose of heat treating protein supplements is to decrease degradability of feed protein in the rumen, and thereby increase the amount of protein that can be digested in the small intestine. High temperatures may cause heat damage to the protein and thus reduce its total tract digestibility, although no negative effects were observed in the current experiment. The amounts of indigestible CP were similar in heat treated HC and the control. Hence, higher amounts of RUP resulted in an increased intestinal digestibility of this fraction, which has also been found in previous studies (Lund et al., 2008; Solanas et al., 2008). Increased in vitro protein digestibility of beans resulting from heat treatment, has been explained by reduced anti-nutritional factors such as tannins and trypsin inhibitor activity (Shimelis and Rakshit, 2005). However, reduced digestibility due to anti-nutritional factors seems to be less of a problem in HC. Hemp protein isolates have been shown to have higher in vitro digestibility (88 to 91%) than soya protein isolate (71%) after pepsin and trypsin digestion (Wang et al., 2008). In the present study, the amount of indigestible CP after the TSP was considerably higher than content of acid detergent insoluble CP (Table 1), which often is used as an indicator of unavailable protein in feeds (Goering et al., 1972).
Results of the current experiment do not allow us to define the optimal temperature for maximizing intestinally available dietary CP and AA. Moist heat treatment at 130°C resulted in the largest amount of RUP with the highest intestinal digestibility. This was the highest temperature that could be achieved in the autoclave but it is possible that higher temperatures could be applied without impairing intestinal digestibility of protein. However, heating rapeseed meal to 145°C was shown to reduce in situ intestinal CP disappearance relative to an untreated control, while there were no negative effects on post-ruminal CP availability when meal was heated to 125°C (McKinnon et al., 1995). Dakowski et al. (1996) found higher intestinal digestibility of CP, total AA and individual AA in rapeseed meal heated to 130°C compared with an untreated control, but temperatures of 140 and 150°C impaired intestinal digestion.
The Maillard, or non-enzymatic browning, reaction involves condensation of amino groups with sugar residues, which may create permanently bound and indigestible N (Van Soest, 1994). The reaction is common during heat treatment of feeds and because specific AA such as lysine may be more readily affected than the protein itself, the protein value may be reduced (Van der Poel et al., 2005). For instance, heat treatment of rapeseed meal resulted in reduced lysine content, as well as available lysine, in the study by Dakowski et al. (1996). In the present study, degradability and digestibility of lysine in the heat treated HC did not differ from that for the total AA, which is in accordance with results found for expander treated feeds (Lund et al., 2008).
In situ intestinal digestibility of CP and total AA have been shown to be similar in several concentrates and, even though some differences exist between individual AA, it has been suggested that intestinal CP digestibility can be used to predict AA digestion (Weisbjerg et al., 1996; Harstad and Prestløkken, 2001). In this study, total AA had higher intestinal digestibility than CP, indicating that the utilization of AA from HC in the small intestine might be underestimated if predicted by CP digestibility. Differences between digestibility of individual AA and total AA within individual HC treatments appeared to be induced by higher treatment temperatures. As discussed previously, values for cystine and methionine need to be confirmed using alternative hydrolysis methods.
IMPLICATIONS
The results of this study provide novel information of the ruminal degradability and intestinal digestibility of crude protein and amino acids in hempseed cake moist heat treated at different temperatures. It increases the knowledge about a relatively unknown protein source and how processing can be used to affect its feed value. The highest temperature used in this study, 130°C, resulted in the largest amount of intestinally available dietary crude protein and amino acids. More studies could confirm the degree of protection as the findings in this study show that moist heat treatment of hempseed cake could be used for extensively shifting site of crude protein and amino acid digestion from the rumen to the small intestine. This may increase the value of hempseed cake as a protein supplement for ruminants, but in vivo verification of the production responses is still required.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the Swedish Farmers Foundation for Agricultural Research for financial support of the project and the SLU Fund for Internationalization of Postgraduate Studies for travel grants for the research visit to The University of Minnesota. We also thank Glen Broderick for comments on the manuscript.