3. Liu RH, Li YH, Jiao LH, Wang XN, Wang H, Wang WH. Extracellular and intracellular factors affecting nuclear and cytoplasmic maturation of porcine oocytes collected from different sizes of follicles. Zygote 2002; 10:253–60.
https://doi.org/10.1017/s0967199402002332


4. Bagg MA, Nottle MB, Armstrong DT, Grupen CG. Relationship between follicle size and oocyte developmental competence in prepubertal and adult pigs. Reprod Fertil Dev 2007; 19:797–803.
https://doi.org/10.1071/rd07018


5. Albuz FK, Sasseville M, Lane M, Armstrong DT, Thompson JG, Gilchrist RB. Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod 2010; 25:2999–3011.
https://doi.org/10.1093/humrep/deq246

6. Cao H, Bian Y, Zhang F, et al. Functional role of Forskolin and PD166285 in the development of denuded mouse oocytes. Asian-Australas J Anim Sci 2018; 31:344–53.
https://doi.org/10.5713/ajas.17.0441

7. Richani D, Wang X, Zeng HT, Smitz J, Thompson JG, Gilchrist RB. Pre-maturation with cAMP modulators in conjunction with EGF-like peptides during in vitro maturation enhances mouse oocyte developmental competence. Mol Reprod Dev 2014; 81:422–35.
https://doi.org/10.1002/mrd.22307

9. Funahashi H, Cantley TC, Day BN. Preincubation of cumulus-oocyte complexes before exposure to gonadotropins improves the developmental competence of porcine embryos matured and fertilized in vitro. Theriogenology 1997; 47:679–86.
https://doi.org/10.1016/s0093-691x(97)00026-5


11. Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology 2004; 62:1186–97.
https://doi.org/10.1016/j.theriogenology.2004.01.011


12. Do L, Shibata Y, Taniguchi M, et al. Melatonin supplementation during in vitro maturation and development supports the development of porcine embryos. Reprod Domest Anim 2015; 50:1054–8.
https://doi.org/10.1111/rda.12607


13. Li Y, Zhang Z, He C, et al. Melatonin protects porcine oocyte in vitro maturation from heat stress. J Pineal Res 2015; 59:365–75.
https://doi.org/10.1111/jpi.12268


15. Steel RGD, Torrie JH. Principles and procedures of statistics, a biometrical approach. 2nd edNew York, USA: McGraw-Hill Kogakusha, Ltd; 1980.
16. Kim EH, Kim GA, Taweechaipaisankul A, et al. Melatonin enhances porcine embryo development via the Nrf2/ARE signaling pathway. J Mol Endocrinol 2019; 63:175–85.
https://doi.org/10.1530/JME-19-0093


18. Töpfer D, Ebeling S, Weitzel JM, Spannbrucker AC. Effect of follicle size on in vitro maturation of pre-pubertal porcine cumulus oocyte complexes. Reprod Domest Anim 2016; 51:370–7.
https://doi.org/10.1111/rda.12688


19. Algriany O, Bevers M, Schoevers E, Colenbrander B, Dieleman S. Follicle size-dependent effects of sow follicular fluid on in vitro cumulus expansion, nuclear maturation and blastocyst formation of sow cumulus oocytes complexes. Theriogenology 2004; 62:1483–97.
https://doi.org/10.1016/j.theriogenology.2004.02.008


20. Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 2002; 296:2178–80.
https://doi.org/10.1126/science.1071965


21. Calder MD, Caveney AN, Westhusin ME, Watson AJ. Cyclooxygenase-2 and prostaglandin E2 (PGE2) receptor messenger RNAs are affected by bovine oocyte maturation time and cumulus-oocyte complex quality, and PGE2 induces moderate expansion of the bovine cumulus in vitro. Biol Reprod 2001; 65:135–40.
https://doi.org/10.1095/biolreprod65.1.135


23. Goetten ALF, Koch J, Rocha CC, et al. Expression profile of key genes involved in DNA repair mechanisms in bovine cumulus cells cultured with bovine serum albumin or fetal calf serum. Reprod Biol 2023; 23:100709
https://doi.org/10.1016/j.repbio.2022.100709


24. Fülöp C, Szántó S, Mukhopadhyay D, et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 2003; 130:2253–61.
https://doi.org/10.1242/dev.00422


25. Matoba S, Bender K, Fahey AG, et al. Predictive value of bovine follicular components as markers of oocyte developmental potential. Reprod Fertil Dev 2014; 26:337–45.
http://doi.org/10.1071/RD13007


26. Zhang X, Jafari N, Barnes RB, Confino E, Milad M, Kazer R. Studies of gene expression in human cumulus cells indicate pentraxin 3 as a possible marker for oocyte quality. Fertil Steril 2005; 83:1169–79.
https://doi.org/10.1016/j.fertnstert.2004.11.030


28. Nakano M, Kato Y, Tsunoda Y. Effect of melatonin treatment on the developmental potential of parthenogenetic and somatic cell nuclear-transferred porcine oocytes in vitro. Zygote 2012; 20:199–207.
https://doi.org/10.1017/S0967199411000190


29. Han J, Wang T, Fu L, et al. Altered oxidative stress, apoptosis/autophagy, and epigenetic modifications in Zearalenone-treated porcine oocytes. Toxicol Res 2015; 4:1184–94.
https://doi.org/10.1039/c5tx00070j

30. Kang JT, Koo OJ, Kwon DK, et al. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J Pineal Res 2009; 46:22–8.
https://doi.org/10.1111/j.1600-079X.2008.00602.x


31. Lin T, Lee JE, Kang JW, et al. Melatonin supplementation during prolonged in vitro maturation improves the quality and development of poor-quality porcine oocytes via anti-oxidative and anti-apoptotic effects. Mol Reprod Dev 2018; 85:665–81.
https://doi.org/10.1002/mrd.23052


32. Shi JM, Tian XZ, Zhou GB, et al. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J Pineal Res 2009; 47:318–23.
https://doi.org/10.1111/j.1600-079X.2009.00717.x


33. Okada K, Krylov V, Kren R, Fulka J. Development of pig embryos after electro-activation and in vitro fertilization in PZM-3 or PZM supplemented with fetal bovine serum. J Reprod Dev 2006; 52:91–8.
https://doi.org/10.1262/jrd.17059


34. Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 2008; 44:280–7.
https://doi.org/10.1111/j.1600-079X.2007.00524.x


35. Zhao XM, Hao HS, Du WH, et al. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J Pineal Res 2016; 60:132–41.
https://doi.org/10.1111/jpi.12290


36. Zhao XM, Min JT, Du WH, et al. Melatonin enhances the in vitro maturation and developmental potential of bovine oocytes denuded of the cumulus oophorus. Zygote 2015; 23:525–36.
https://doi.org/10.1017/S0967199414000161


40. Zhao XM, Wang N, Hao HS, et al. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J Pineal Res 2018; 64:e12445
https://doi.org/10.1111/jpi.12445




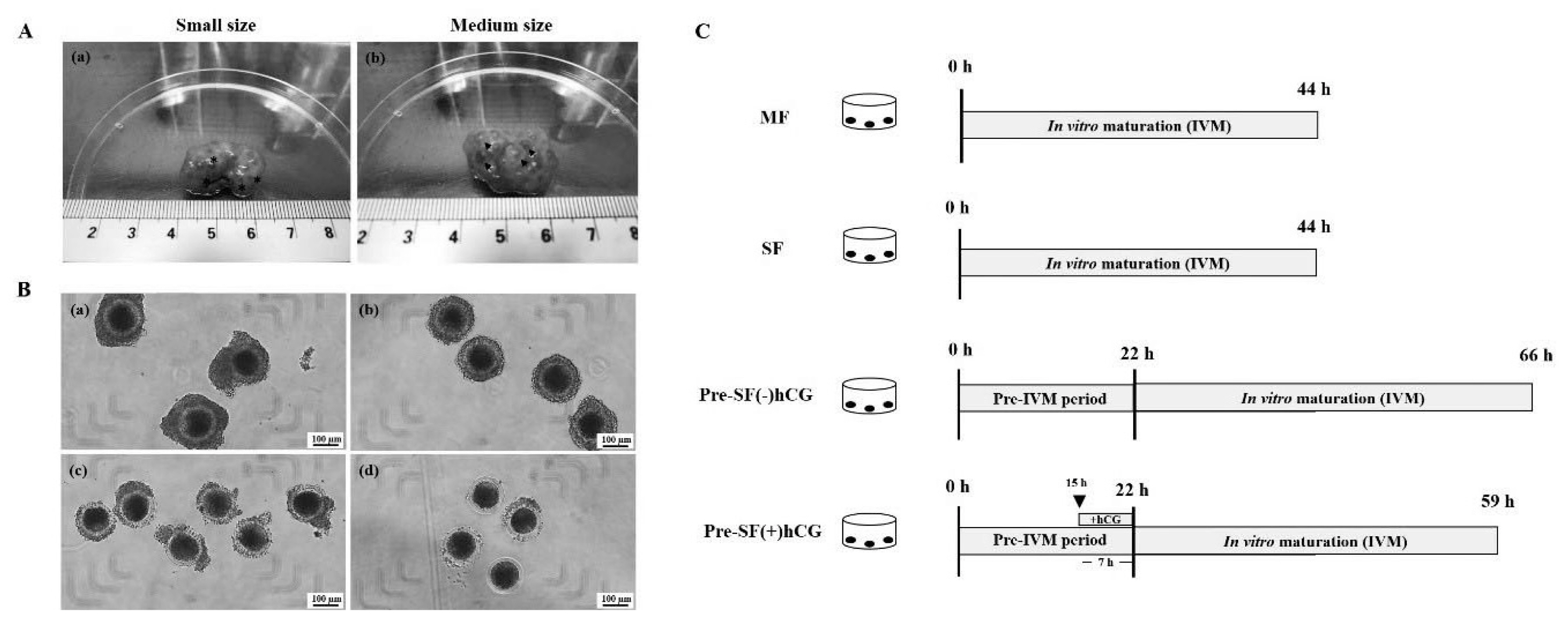
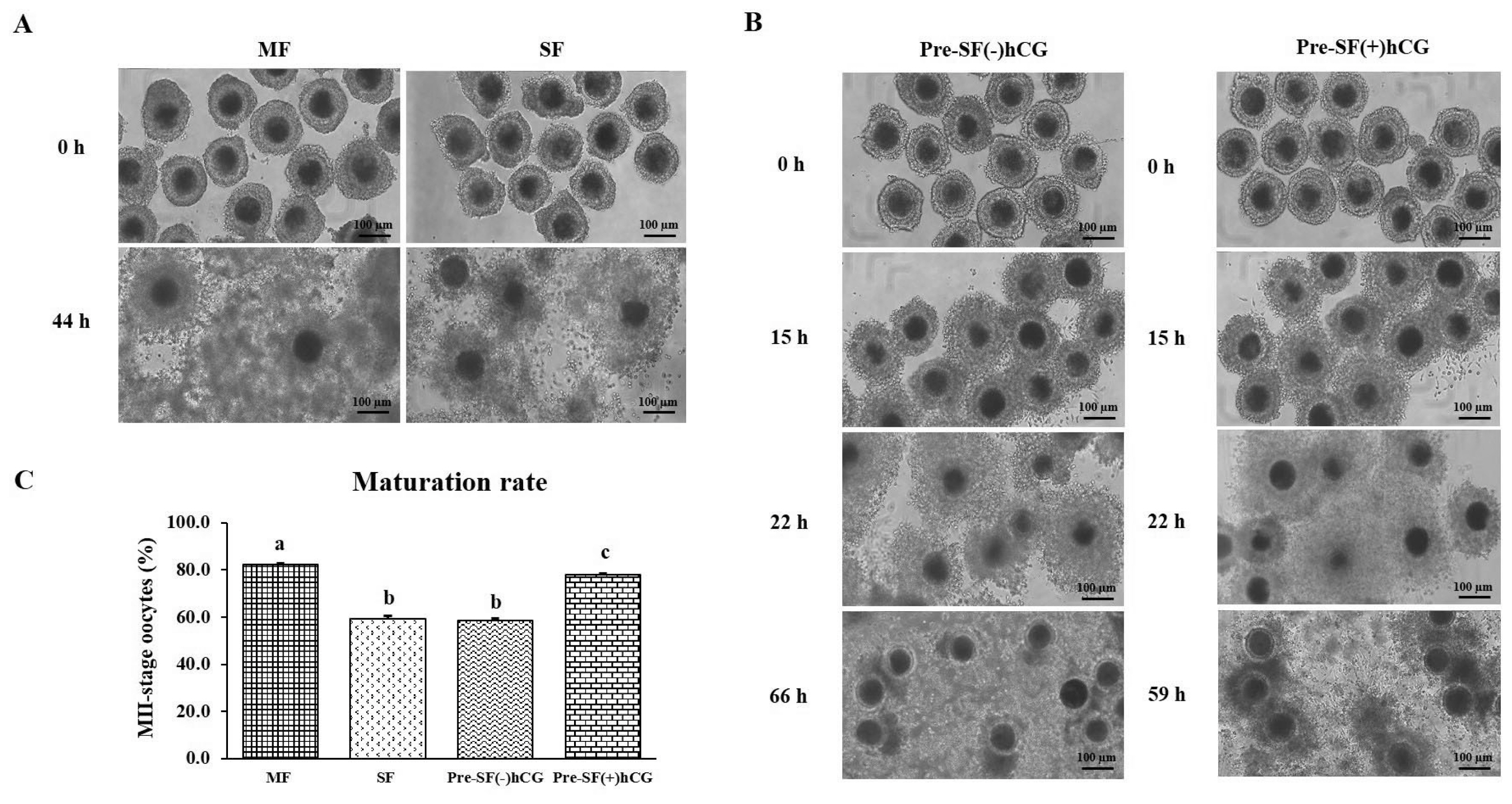
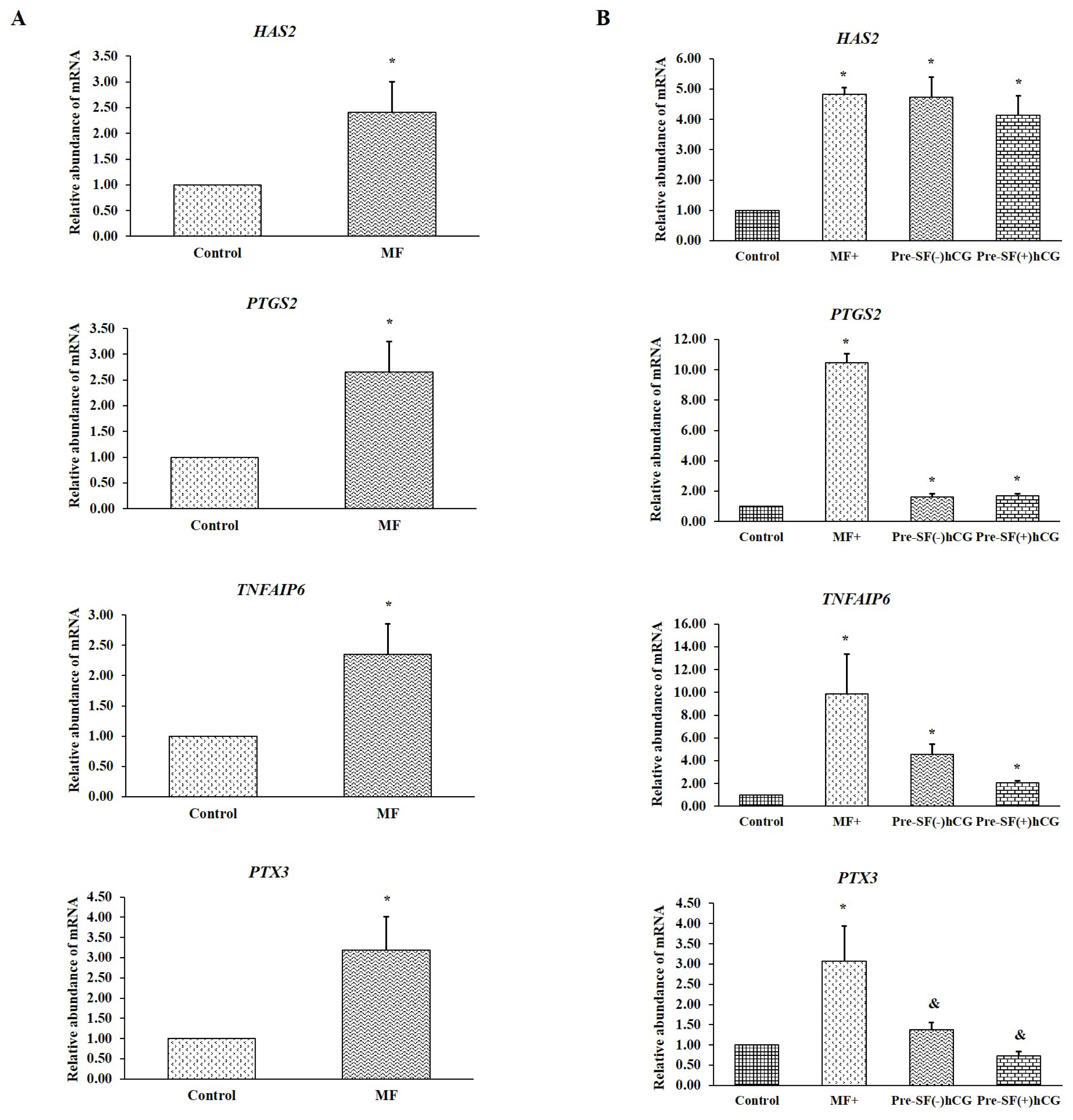
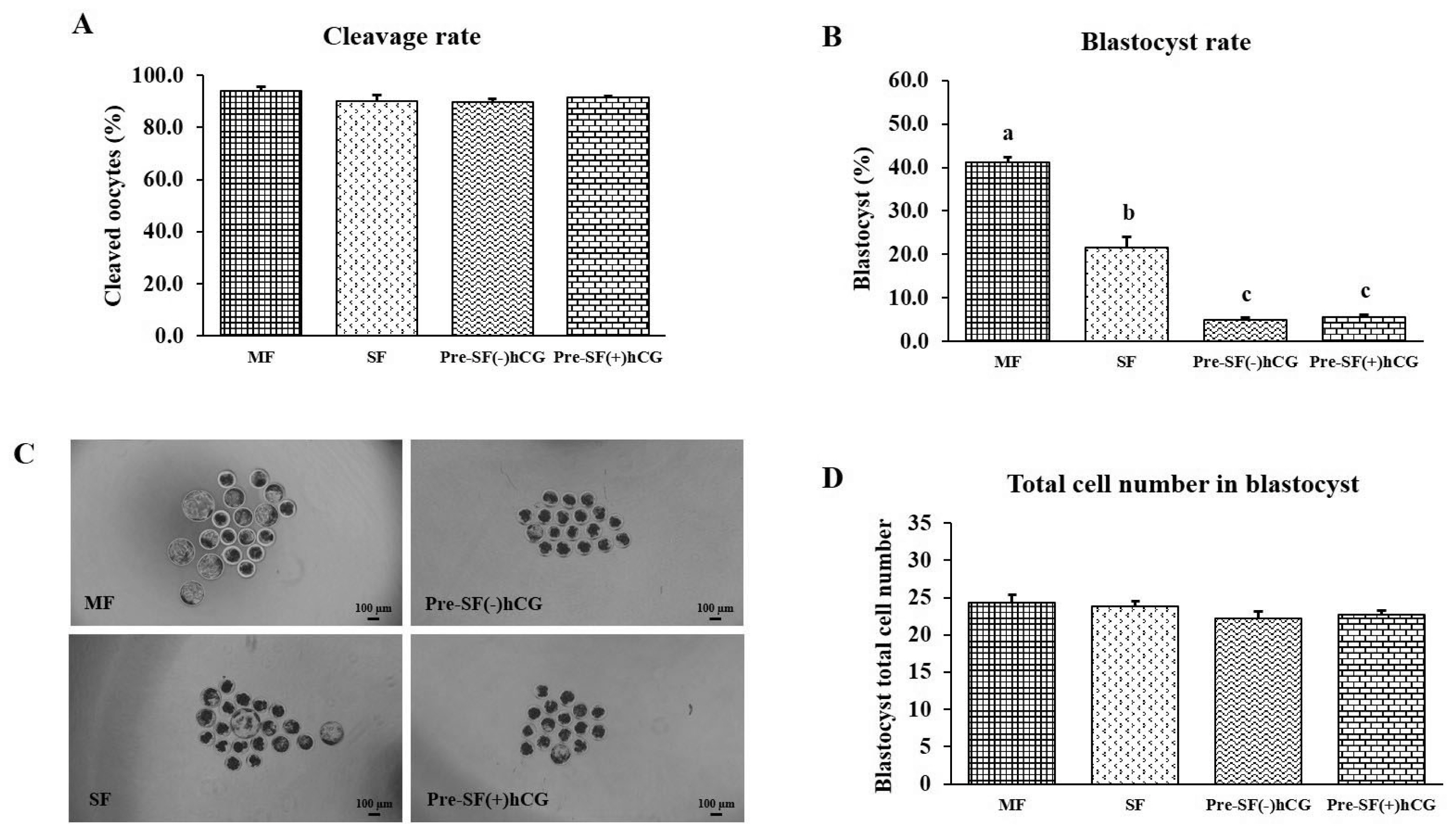






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





