 |
 |
| Anim Biosci > Volume 35(7); 2022 > Article |
|
Abstract
Objective
Methods
Results
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
This work was supported by Suranaree University of Technology (SUT), Thailand Science Research and Innovation (TSRI), National Science, Research and Innovation Fund (NSRF), and the National Research Council of Thailand (NRCT) (SUT3-303-60-24-09).
The authors would like to acknowledge the staff of FTIR team (beam line 4.1: Infrared Spectroscopy and Imaging) from Synchrotron Light Research Institute (Public Organization) in Thailand for their technical suggestions. We would also like to express our sincere thanks to Pascal Mermillod from Reproductive Physiology and Behavior unit, National Institute of Agronomical Research (INRA), France for his guiding and proofreading this manuscript with the support of the Siam Huber Curien grant n° 42879QC.
Figure 1
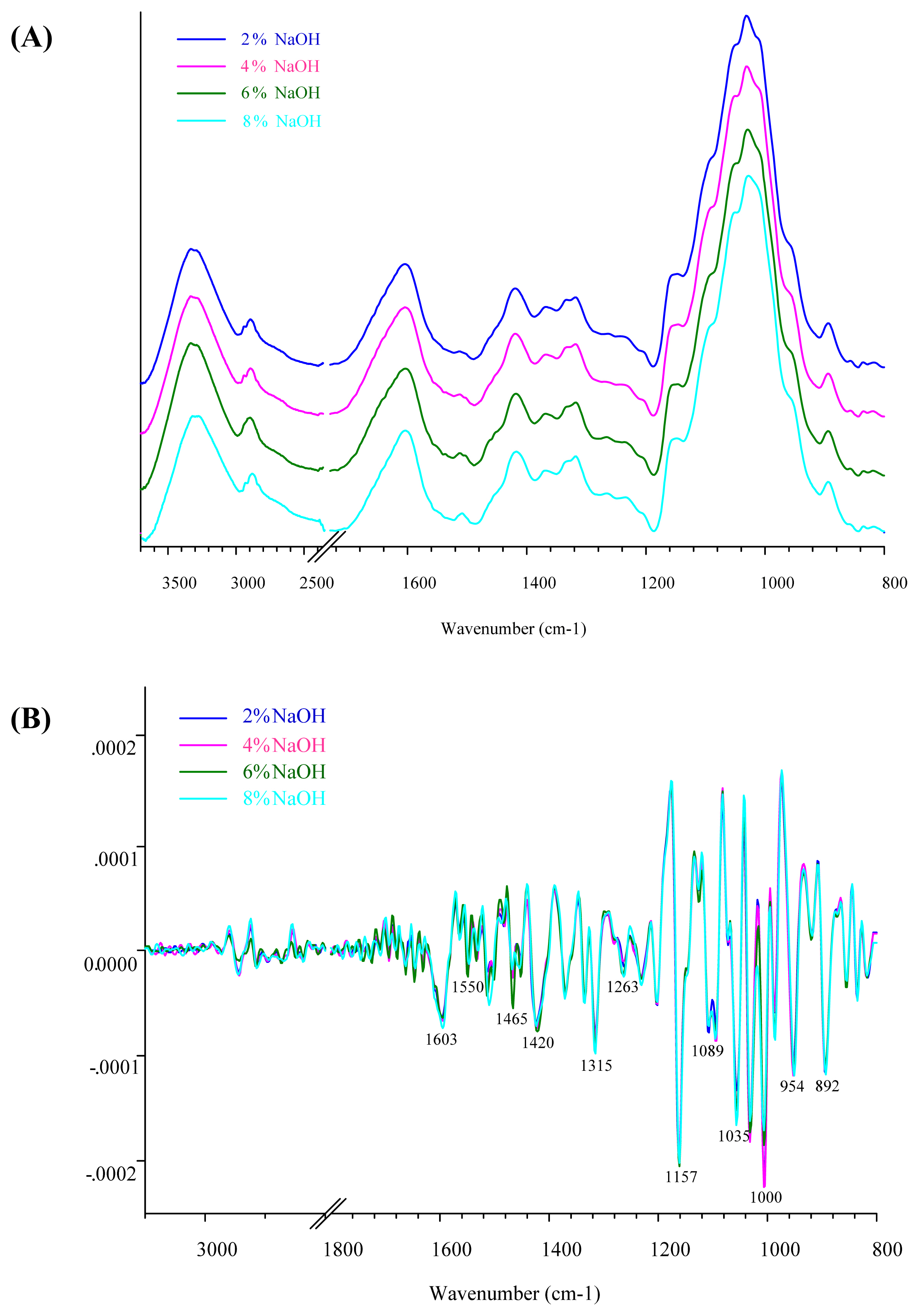
Figure 2
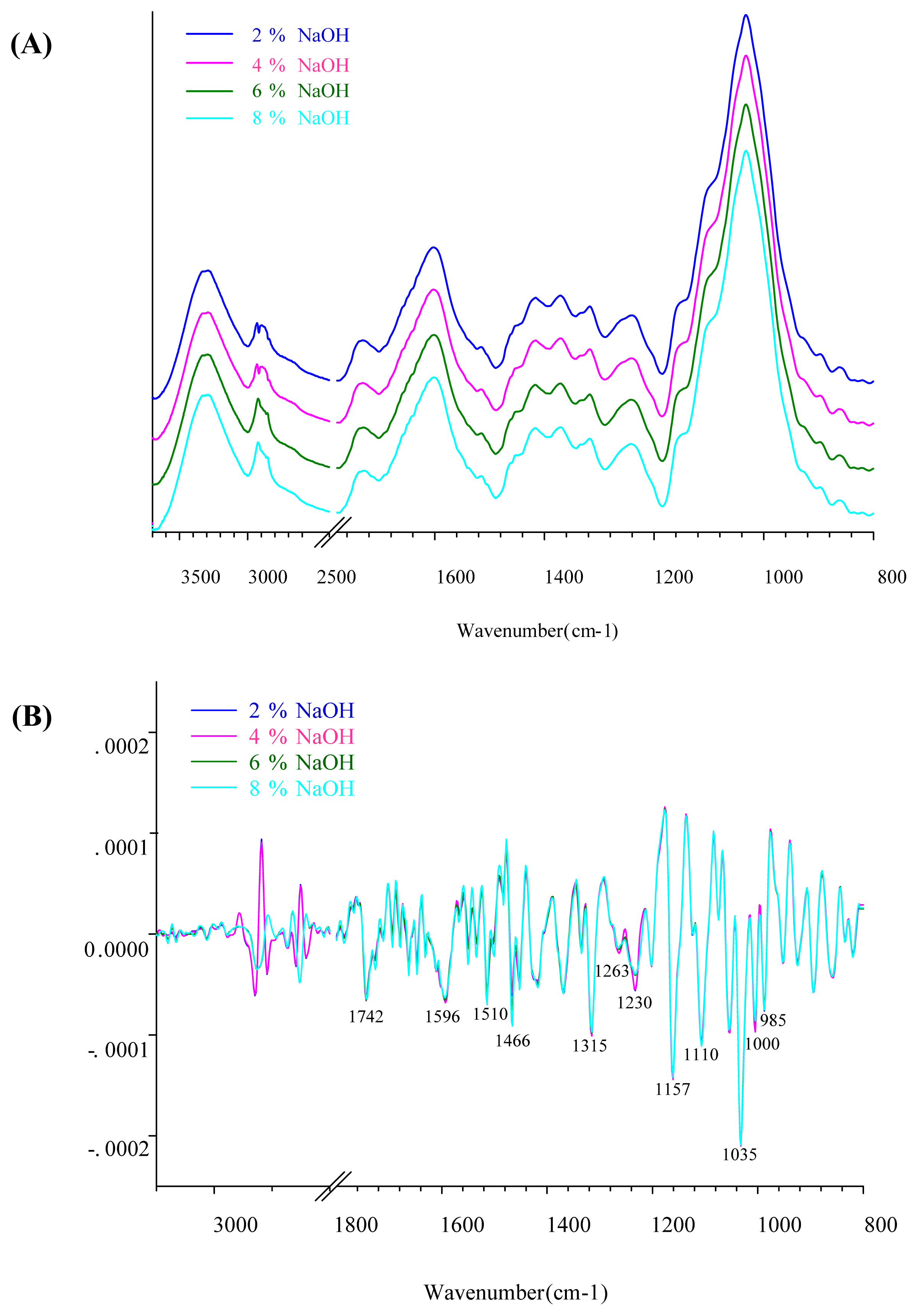
Figure 3
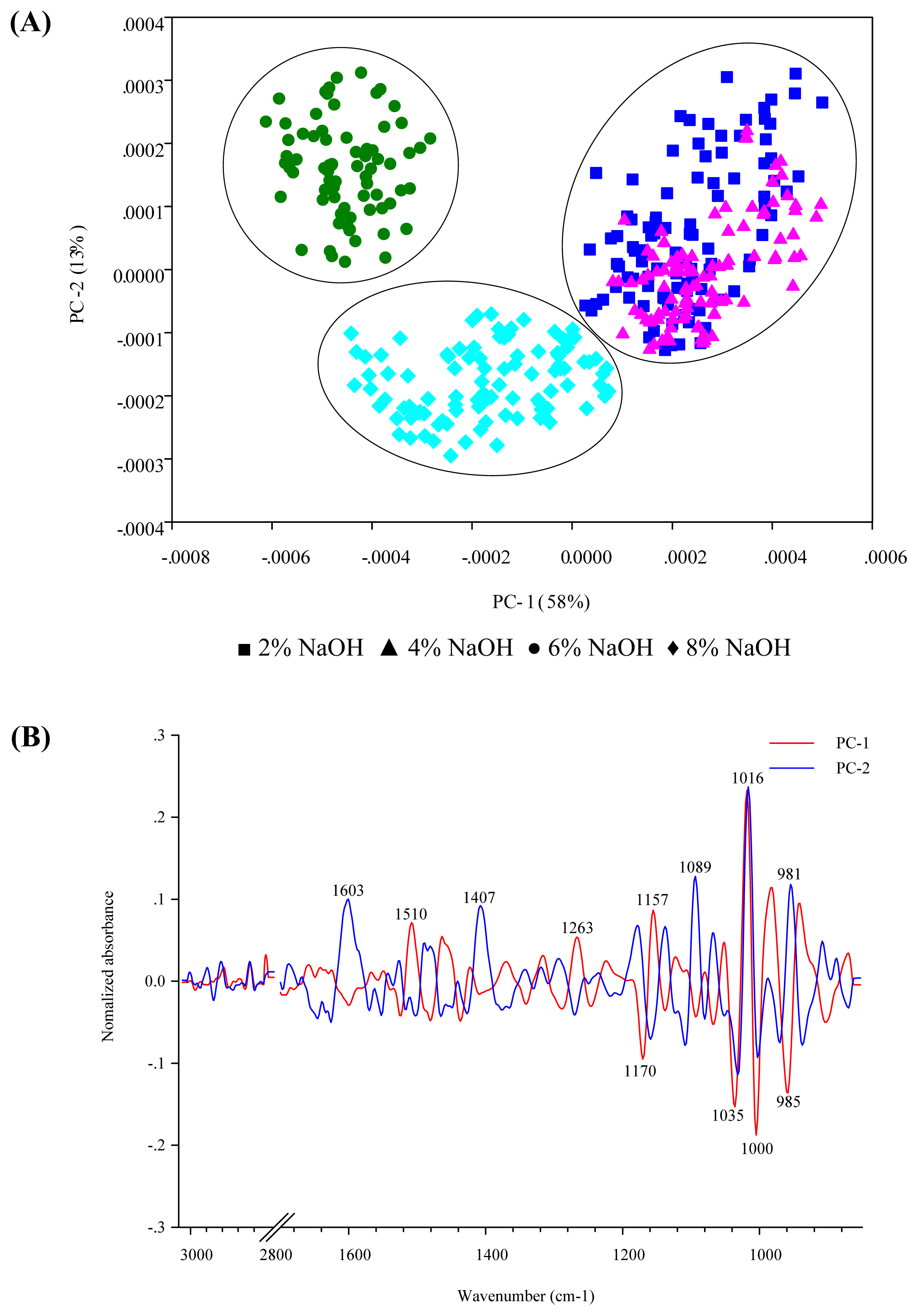
Figure 4

Table 1
Table 2
| Item | Concentration of NaOH levels | SEM | p-values | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| 2% | 4% | 6% | 8% | ANOVA | Linear1) | Quadratic1) | Cubic1) | ||
| Cassava pulp extracted dietary fiber | |||||||||
| Total dietary fiber | 23.60b | 24.29b | 27.93a | 27.60a | 0.57 | 0.001 | 0.001 | 0.285 | 0.032 |
| Soluble dietary fiber | 3.65 | 2.57 | 3.19 | 2.84 | 0.19 | 0.501 | 0.501 | 0.188 | 0.365 |
| Insoluble dietary fiber | 19.95b | 21.72ab | 24.73a | 24.76a | 0.63 | 0.001 | 0.001 | 0.117 | 0.089 |
| Cassava distiller’s dried grains extracted dietary fiber | |||||||||
| Total dietary fiber | 22.85b | 25.12a | 25.33a | 25.35a | 0.30 | <0.001 | <0.001 | <0.001 | 0.009 |
| Soluble dietary fiber | 2.38 | 2.59 | 2.33 | 2.32 | 0.06 | 0.313 | 0.368 | 0.356 | 0.152 |
| Insoluble dietary fiber | 20.46b | 22.53a | 23.00a | 23.03a | 0.31 | <0.001 | <0.001 | <0.001 | 0.186 |
Table 3
| Wavenumber (cm−1) | Assignments | Reference |
|---|---|---|
| 2,800 – 3,000 | C–H stretching (aliphatic compounds) |
Lammers et al [22] Abidi et al [27] Pouzet et al [12] |
| 1,600 – 1,680 | O–H bending of adsorbed water |
Abidi et al [27] Pouzet et al [12] |
| 1,514, 1,595 | C–H deformation of lignin | Lammers et al [22] |
| 1,300 – 1,500 | C–H bending (crystalline versus amorphous structure of cellulose) |
Abidi et al [27] Ying et al [28] |
| 1,200 – 1,260 | C–O stretching of hemicellulose |
Corredor et al [23] Abidi et al [27] Cheikh Rouhou et al [29] |
| 1,035 – 1,160 | C–O–C glycoside in cellulose, C–O vibration of crystalline cellulose, C–O stretching and C–C stretching of cellulose | Corredor et al [23] |
|
Abidi et al [27] Ying et al [28] Cheikh Rouhou et al [29] |
||
| 980 – 1,000 | C–O and ring stretching modes, C–O stretching of starch |
Lammers et al [22] Abidi et al [27] |
| 800 – 960 | Vibration of the pyranose ring, glucose ring stretch |
Lammers et al [22] Corredor et al [23] |
Table 4
| Item | Concentration of NaOH levels | SEM | p-values | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| 2% | 4% | 6% | 8% | ANOVA | Linear1) | Quadratic1) | Cubic1) | ||
| Cassava pulp extracted dietary fiber | |||||||||
| C–H stretching | 5.33 | 6.22 | 4.52 | 5.08 | 0.38 | 0.486 | 0.486 | 0.832 | 0.180 |
| O–H bending of adsorbed water | 8.86 | 8.87 | 7.79 | 8.52 | 0.17 | 0.065 | 0.116 | 0.237 | 0.052 |
| C–H deformation of lignin | 0.66 | 0.79 | 0.75 | 0.73 | 0.04 | 0.612 | 0.595 | 0.272 | 0.574 |
| C–H bending of crystalline cellulose | 18.20bc | 17.06c | 19.54a | 18.57ab | 0.27 | 0.001 | 0.018 | 0.769 | <0.001 |
| C–O stretching of hemicellulose | 5.40a | 5.04b | 5.41a | 5.32a | 0.05 | 0.006 | 0.686 | 0.046 | 0.001 |
| C–O–C glycoside, C–O and C–C stretching of cellulose | 25.28b | 25.51b | 26.56a | 25.69ab | 0.17 | 0.015 | 0.060 | 0.046 | 0.029 |
| C–O stretching of starch | 17.96 | 18.21 | 18.75 | 19.11 | 0.24 | 0.346 | 0.082 | 0.905 | 0.831 |
| Vibration of the pyranose ring | 18.15 | 17.97 | 16.99 | 17.15 | 0.23 | 0.178 | 0.055 | 0.679 | 0.318 |
| Cassava distiller’s dried grains extracted dietary fiber | |||||||||
| C–H stretching | 3.56ab | 3.89a | 2.43bc | 2.10c | 0.25 | 0.007 | 0.002 | 0.345 | 0.087 |
| O–H bending of adsorbed water | 7.52 | 6.84 | 6.98 | 6.41 | 0.18 | 0.134 | 0.040 | 0.878 | 0.315 |
| C–H deformation of lignin | 1.03 | 0.68 | 0.81 | 0.87 | 0.08 | 0.528 | 0.648 | 0.252 | 0.506 |
| C–H bending of crystalline cellulose | 29.03a | 27.30ab | 27.71ab | 26.38b | 0.37 | 0.049 | 0.015 | 0.746 | 0.189 |
| C–O stretching of hemicellulose | 5.60 | 6.02 | 5.80 | 5.34 | 0.12 | 0.222 | 0.330 | 0.081 | 0.707 |
| C–O–C glycoside, C–O and C–C stretching of cellulose | 20.57b | 22.58a | 21.95a | 22.28a | 0.26 | 0.004 | 0.008 | 0.024 | 0.033 |
| C–O stretching of starch | 22.67c | 23.23bc | 24.36ab | 25.64a | 0.35 | 0.001 | <0.001 | 0.356 | 0.807 |
| Vibration of the pyranose ring | 10.03b | 9.44b | 9.94b | 10.98a | 0.18 | 0.003 | 0.005 | 0.004 | 0.593 |
REFERENCES
- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print





