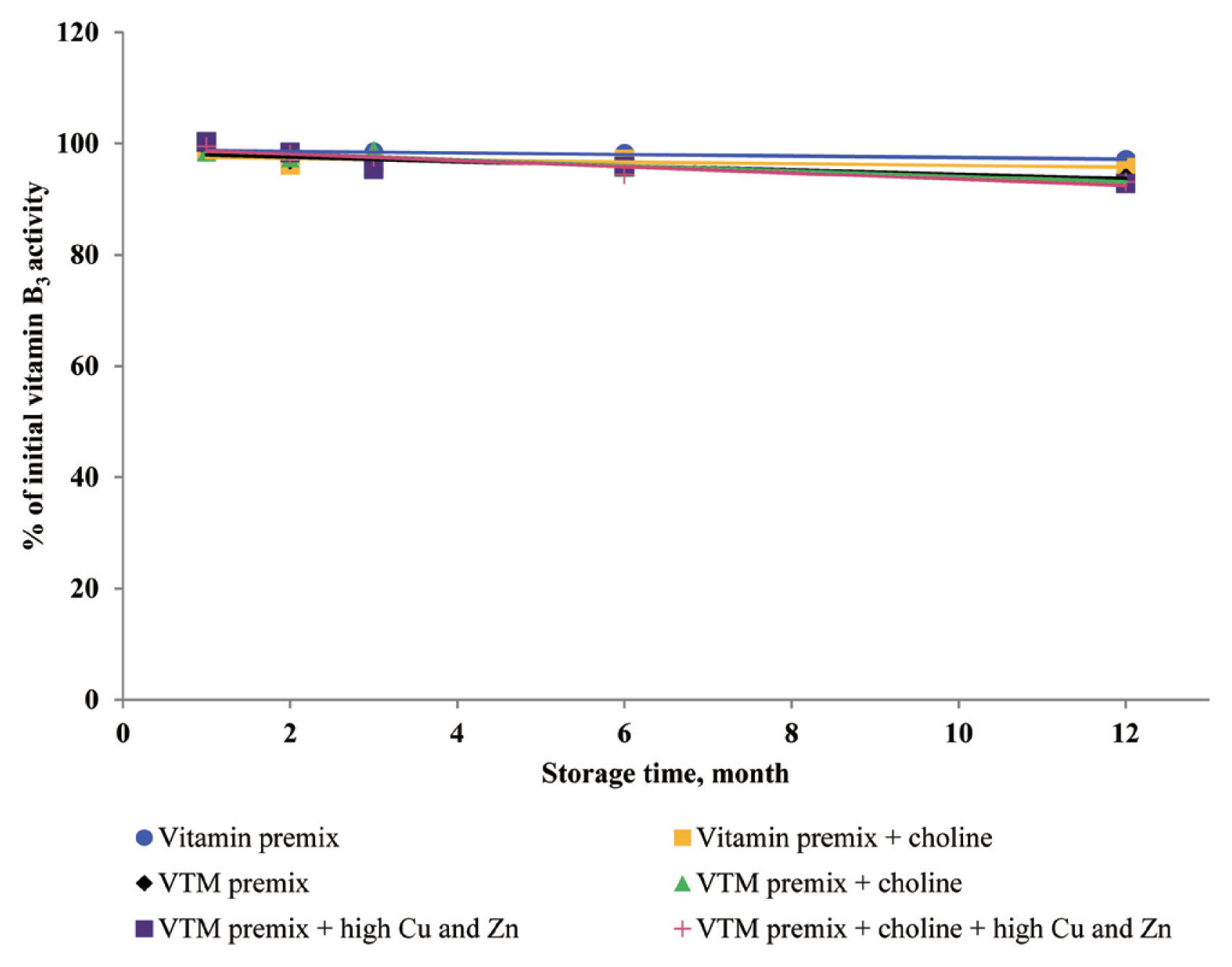
Extended storage time affected the stability of vitamins
Determination of vitamin stability before and after storing is necessary to assess the final amount of actual vitamin that will reach the end-user and to calculate what amount should be added to any feed matrix. Storing vitamins for long periods has been considered to negatively affect vitamin activity in vitamin or VTM premixes. However, the results of our study indicate that long-term storage of vitamin premixes had little influence upon VA, VE, and VB
3 concentrations. The results from our study agreed with previous studies on vitamin stability. Coelho [
8] reported that the average loss per month of VA, VE, and VB
3 after storage was 2.9%, 2.7%, and 2.7%, respectively. Because most manufactures of VA producers stabilize VA using the method of sealing within a physical matrix, generally arabic gum or gelatin [
2,
18]. In addition, in the production of commercially-available VA and VE, their hydroxy group is protected by the formation of an ester, as in ╬▒-tocopherol acetate. The obtained ╬▒-tocopherol acetate is resistant to oxygen, since it lacks double bonds [
3,
9]. In the present study, the retention of niacin was more than 90% after 1 year of storage, which is consistent with Zhuge and Klopfenstein [
19], who reported that the retention rate of VB
3 during storage was 91% to 96% during 27 weeks storage. This was not surprising to us because VB
3 has been reported to be the most stable among the B vitamins when added to feed or premixes [
19] owing to its a stable molecular structure, which reduces its oxidation during storage. Coelho [
8] reported that the loss of commercial fat-soluble vitamins after one month of storage was less than 10%; after six months of storage, its loss rate was 10% to 60%. Shurson et al [
20] reported that vitamin activity in vitamin and VTM premixes decreased with prolonged storage time, but stability of VE, VB
2, VB
3, VB
5, and VB
6 are higher than that of other vitamins, which is in line with our results.
Addition of choline chloride affected vitamin stability
Choline chloride has been reported to significantly affect vitamin activity [
8ŌĆō
10]. Choline is considered a stress agent that affects vitamins that dissolve easily in water because it is hygroscopic, and can attract moisture to vitamin or VTM premixes [
3]. The concentration of choline chloride in feed is usually higher than the micro-ingredient level, and problems related to both physical and chemical properties can be expected when choline is added to vitamin or VTM premixes [
8ŌĆō
10]. After six months of storage, the loss of vitamin in a vitamin premix without choline chloride was 1% to 5% [
8]; with choline chloride in the premix, the vitamin loss up to 32% after six months of storage [
8]. In addition, the negative effects of choline chloride on vitamins in a VTM premix were more significant. After six months of storage, the loss of vitamin in a VTM premix without choline chloride was 12% to 30% [
8]. However, in a VTM premix with choline chloride, the loss after six months was 23% to 52% [
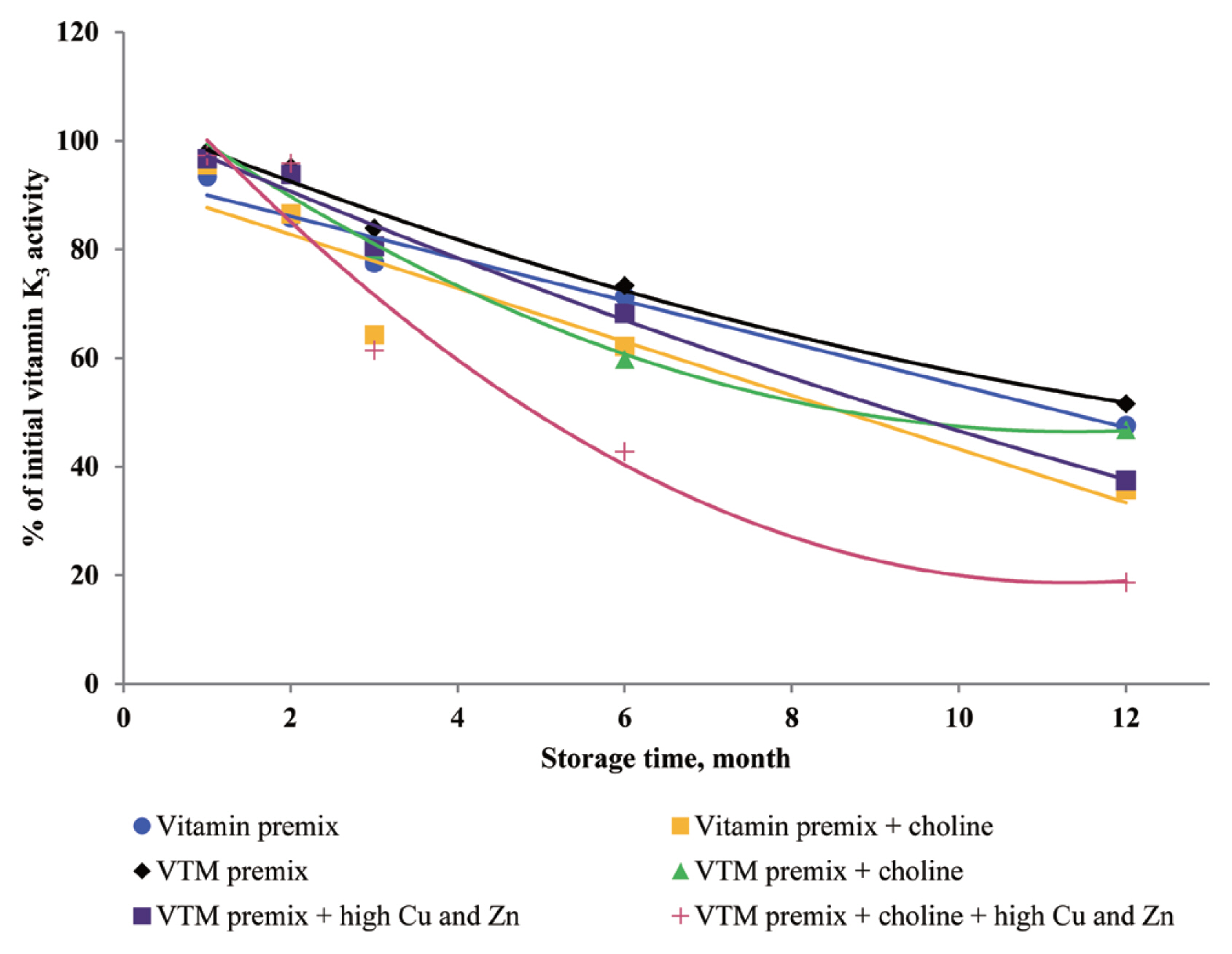
8]. Vitamin K is known for its contribution to the blood clotting or coagulation process. The VK
3 was more stable in vitamin premix without choline than in the vitamin premix with choline during one-year storage, which was consistent with Tav─Źar-Kalcher and Vengu┼Īt [
10]. Results from the present study suggest that loss of VK
3 activity was approximately 60% in two vitamin premixes. Our results are similar to the average monthly losses reported by Coelho [
8]. In the current study, choline chloride is a significant factor affecting the loss of VK
3 activity. Menadione (VK
3) is the form of vitamin K that is used in animal nutrition. It is not utilized in pure form in premix plants but was formulated with sodium bisulfite and derivatives. The most common menadione compound used in the industry is the water-soluble salt, menadione sodium bisulfite. It was reported that VK
3 was very sensitive to moisture and trace minerals, and choline chloride was particularly destructive to VK
3 [
10]. Furthermore, supplemented choline chloride in vitamin or VTM premixes increased leaching of VK
3 and prolonged oxidation-reduction reactions [
9,
10]. The inclusion of choline chloride in a VTM premix contributes to VA, VK
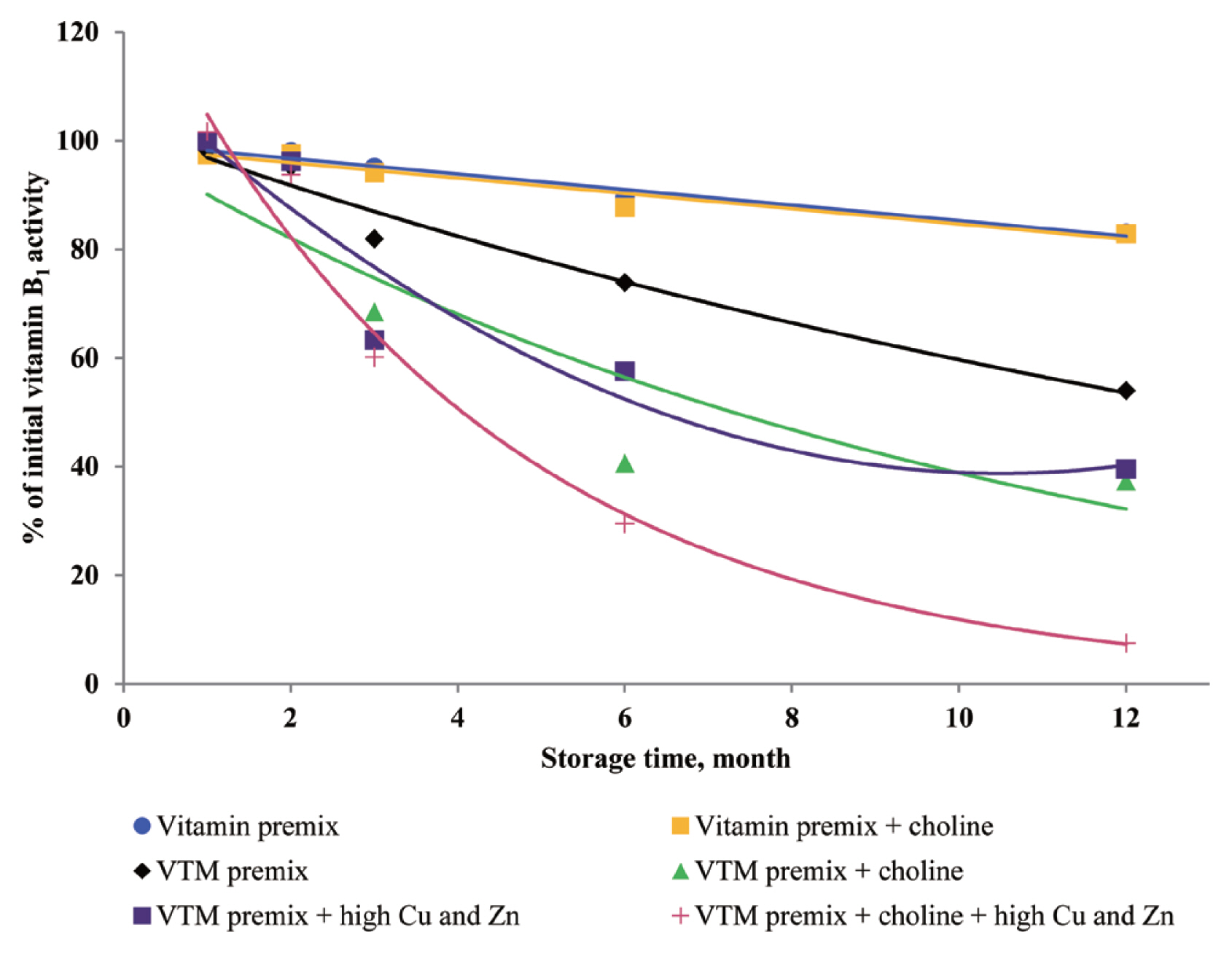
3, VB
1, and VB
2 instability during storage. Stability data published by BASF 1994, cited by Whitehead [
21], showed VA, VK
3, VB
1, and VB
2 loss of 15%, 36%, 30%, and 5% after one month and 42%, 100%, 73%, and 44% after six months of storage in VTM premixes containing choline chloride. Besides, we observed that loss of VA, VK
3, VB
1, and VB
2 was lower than that reported by Whitehead [
21]. The reason may be that the vitamin manufacturing industry has developed products with improved stability. In the present study, we used thiamine mononitrate as VB
1 source, it is used more often in feed because of its higher stability compared to thiamine hydrochloride [
3,
8]. In addition, the commercial form of VA and VB
2 is spray-dried processing, which usually provides improved stability during storage and transportation.
Excessive Cu and Zn elements in VTM premix affected vitamin stability
The VTM premixes are the most common dietary supplements. In commercial conditions, feeding piglets with high concentrations of Zn and Cu stimulates their average daily gain, decreases the feed conversion ratio, improves the digestibility of dietary nutrients and growth performance, and decreases the incidence of diarrhea [
6,
7]. However, minerals in premixes are usually in an inorganic form: sulfates, chlorides, oxides, etc. Different metal compounds have different capability of catalytic oxidation reaction [
8]. In the present study, high levels of CuSO
4 (more than 20,000 mg/kg of Cu in premix to promote growth) and ZnO (more than 225,000 mg/kg of Zn in premix to decrease the incidence of diarrhea) in VTM premixes resulted in the degradation of vitamins. Vitamin stability is reduced in the presence of certain trace minerals [
8,
9,
20]. In our study, blending vitamins with trace minerals to form VTM premixes increased the loss of vitamin activity during prolonged storage periods. These trace minerals in premix can catalyze the generation of free radicals, thereby oxidizing antioxidants during storage. Certain metal-catalyzed destruction of vitamins in the feed matrix has been reviewed previously to a limited extent [
22]. Trace minerals vary in their redox potential: Cu, Fe, and Zn are the most reactive, and Se, I, and Mg are less reactive minerals [
8,
22]. Reactive trace minerals reduce vitamin activity by oxidizing the vitamins. First, the metallic-like nature of trace minerals reduces the crystals of vitamins to smaller particles by eroding their protective coating. The smaller particles provide increased surface areas of vitamins for reactions between vitamin particles and trace mineral particles.
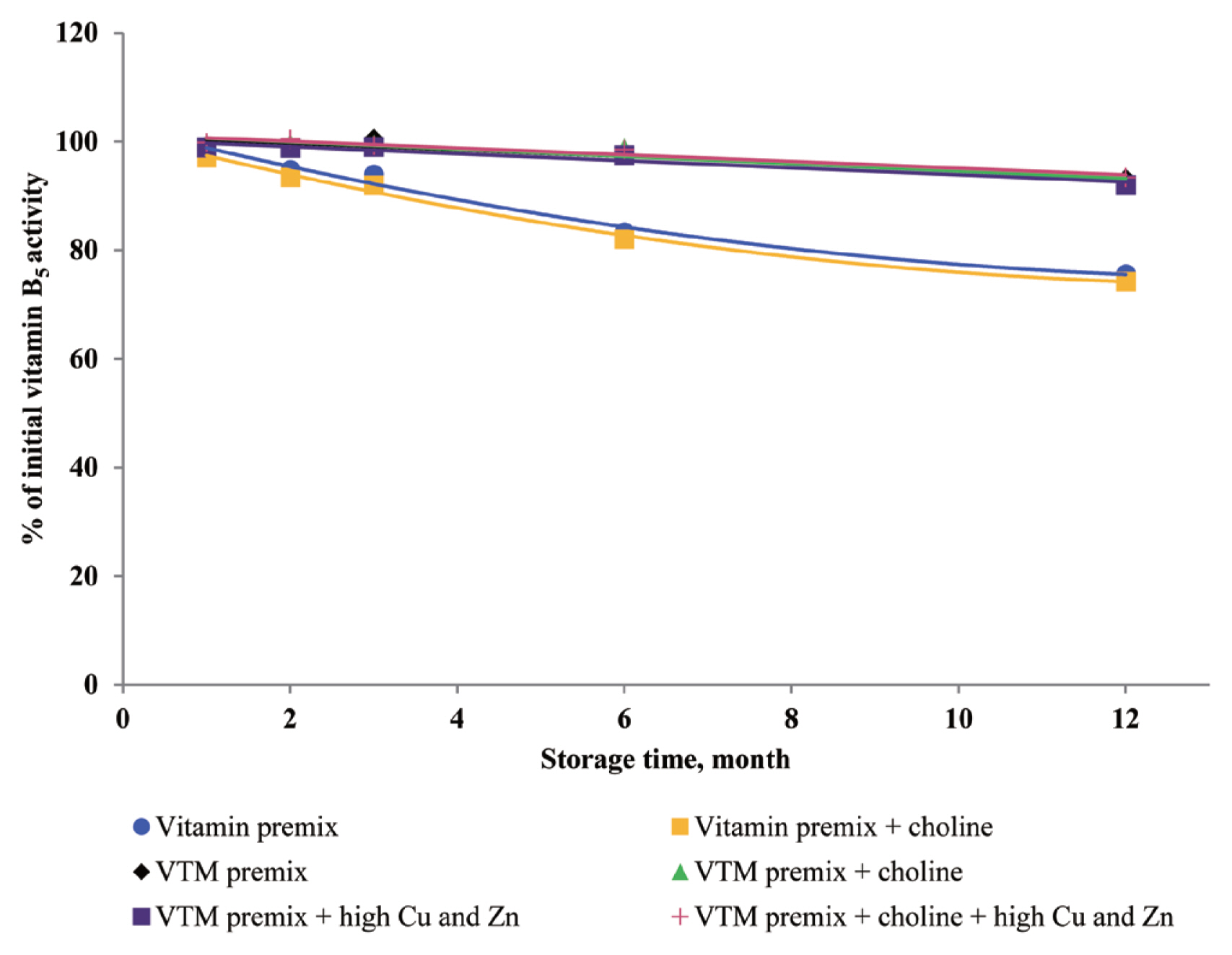
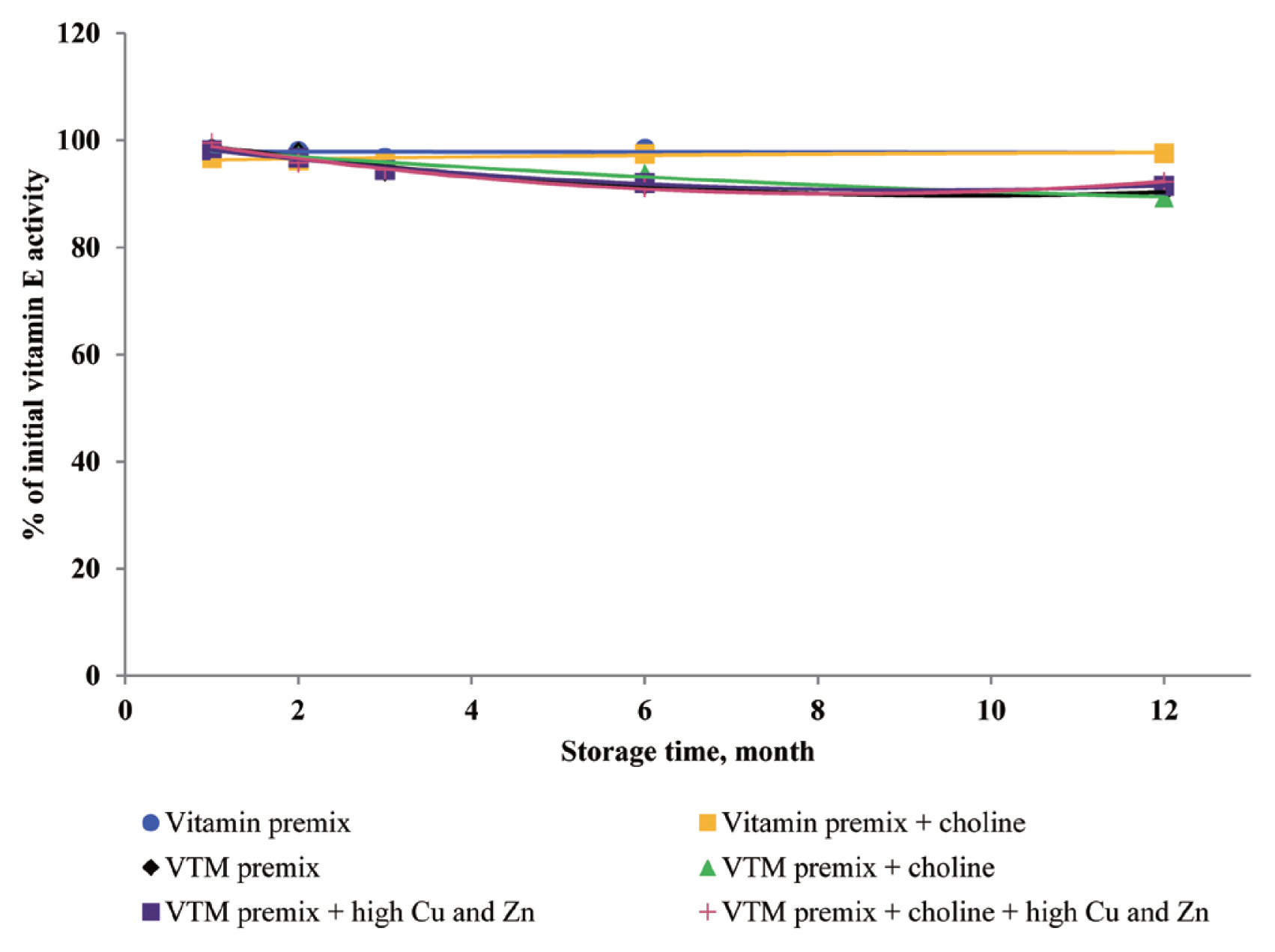
Dove and Ewan [
23] reported that a high concentration of Cu (250 mg/kg feed) or Zn (1,000 mg/kg feed) increased vitamin loss. Redox-active transition metals, such as Cu, can serve as catalysts for the oxidation of organic compounds. Lu et al [
24] reported that a high concentration of Cu sulfate promoted the undesirable oxidation of VE in feeds. Intriguingly, we did not find significant effects on VE content in the four VTM premixes during long-term storage. There are two significant factors that might contribute to achieve this positive characteristic. First, supplementation of VE are generally given in the form of all-rac-╬▒-tocopheryl acetate in which the reactive hydroxyl group of ╬▒-tocopherol is esterified; rendering the molecule more stable than the free phenol form, and second, production of VE provides a physical barrier to reduce surface contact with pro-oxidant agents such as Zn or Cu. On the other hand, we observed long-term storage of VTM premix with high concentrations of Cu and Zn had no effect on VB
3 and VB
5 concentrations. The results from our study confirm previous studies on the stability of these vitamins. Shurson et al [
20] reported recovery of VB
3 after 120 days of storage in a vitamin stock was 96.68%, 86.04% in vitamin premix, and 87.04% in VTM premix. Zhuge and Klopfenstein [
19] also reported that VB
3 was considerably more stable than other vitamins; at the end of 27 weeks storage, VB
3 in premixes with or without mineral retained 91% and 96%, respectively. The VB
3 is probably the most stable of the water-soluble vitamins when added to feed or premixes, being little affected by heat, oxygen, moisture, or light [
3,
11]. However, there are no reports on the mechanism of resistance to degradation or subsequent degradation products. The VB
5, pantothenic acid, is a constituent of coenzyme A, which both act as carriers of acyl groups and activators of carbonyl groups in many metabolic processes [
2,
3]. Pure VB
5 is a viscous, hygroscopic, and chemically-unstable oil. In premixes, this vitamin is commonly added as calcium pantothenate, a soluble and stable solid. The calcium salt is preferred to the sodium salt, as solid forms of the sodium salt are much more hygroscopic. Similar to niacin, there were no significant influences of choline and high concentrations of Cu and Zn on pantothenic acid retention in VTM premixes. But storage time was a significant factor affecting the loss of VB
5 under ambient temperature and relative humidity [
3]. According to the previous results [
20], there was no significant difference in the stability of VB
5 after 120 days of storage at vitamin stock, vitamin premix and vitamin-inorganic trace minerals premix. A similar result was reported by Coelho [
8]. Furthermore, it was reported that pantothenic acid was relatively stable to heat, oxygen, and light [
8]. The stability of pantothenic acid is due to the presence of the carboxylic acid group to form two hydrogen bonds between a pair of molecules [
25]. Furthermore, the amide group and two methyl groups in the aliphatic chain of pantothenic acid contribute to its stability.
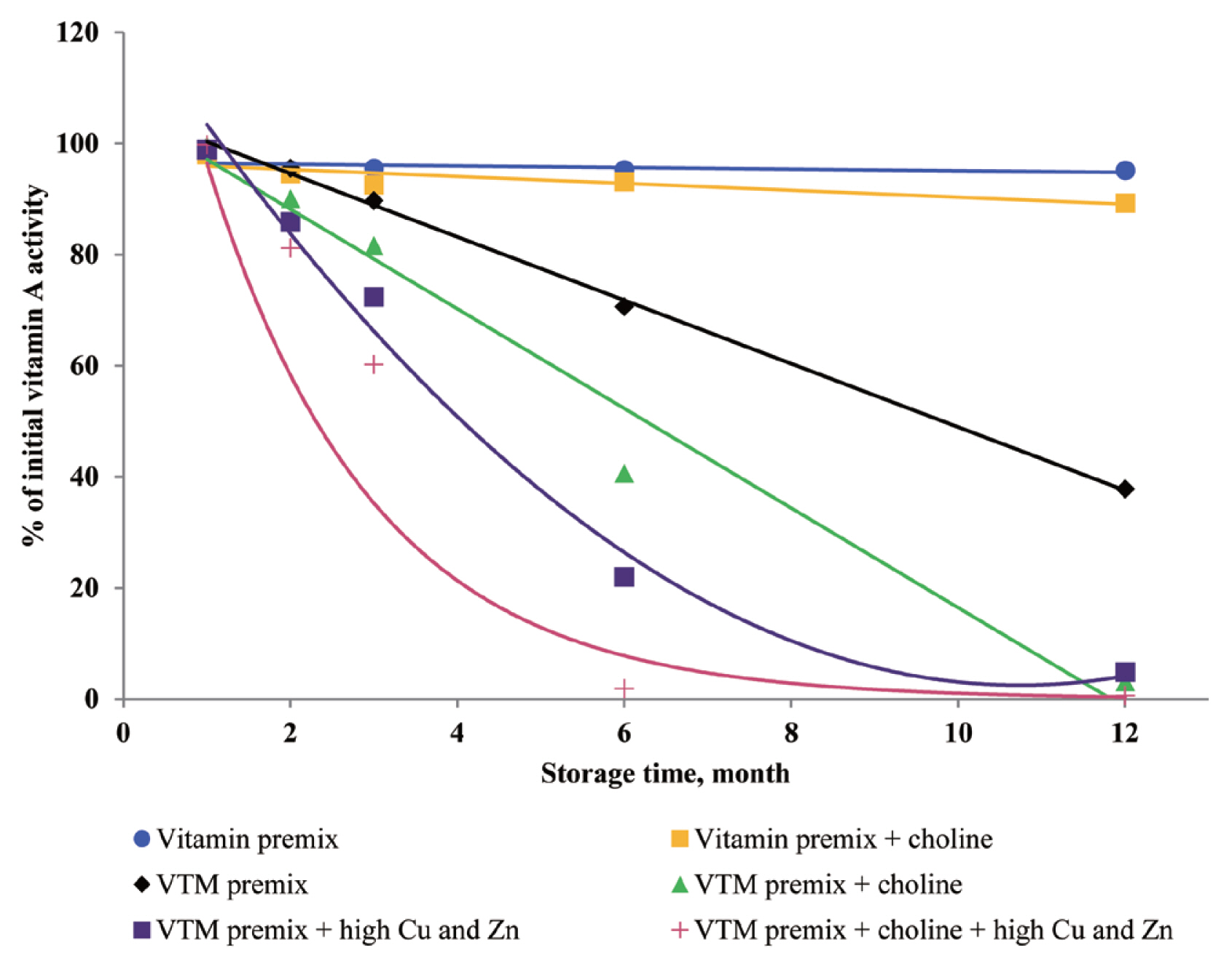
In the current study, the supplementation of high concentrations of Cu and Zn significantly reduced the concentrations of VA, VD
3, VK
3, VB
1, VB
2, and VB
6 in VTM premixes during storage. The reason can be explained that the presence of Zn and Cu in premix can speed up vitamin degradation [
3,
8,
9], these minerals can catalyze the generation of free radicals which can oxidize vitamins in VTM premixes. Also, the results from our study confirm previous studies on vitamin stability in VTM premix. Shurson et al [
20] reported that the stability of VA, VK
3, VB
1, and VB
6 in premixes was influenced by the presence of trace minerals. These results were in line with previous study. Yang et al [
9] reported that stability of VA, VB
1, and VB
6 was affected by trace minerals, and the premixes containing high levels of Cu and Zn was more susceptible to oxidative reaction of vitamins. Factors that reduce VA activity are atmospheric oxygen, light, heat and other oxidizing agents [
3]. Manan et al [
26] looked at the stability of VA in the presence of Cu and Zn; the results showed that mineral fortification reduced VA stability by 36%. Pinkaew et al [
27] reported the stability of VA in the presence of ZnO; the results showed that mineral fortification reduced the VA stability by 13.4%. The chemical structure of VA is an unsaturated monohydric alcohol with 20 carbon atoms, consisting of a cyclohexane ring linked to a polyunsaturated chain that terminates in an alcohol group. The five conjugated double bonds in the configuration of VA are easy points of attack for oxygen. The oxidation of the alcohol end group of VA results in the formation of retinal or all-trans retinaldehyde, which can be further oxidized to all-trans retinoic acid. Besides, the structure of VB
1 can help to understand its instability during storage. The methylene bridge connecting the pyrimidine and thiazole moiety can easily be broken down by oxidizing ingredients [
25]. In addition, the VB
6 comprises a group of three related compounds: pyridoxine, pyridoxal, and pyridoxamine. Pyridoxine is commonly used for feed because pyridoxine is more stable than either pyridoxal or pyridoxamine [
3,
8], but pyridoxine was sensitive to light, particularly in neutral and alkaline solutions. The VB
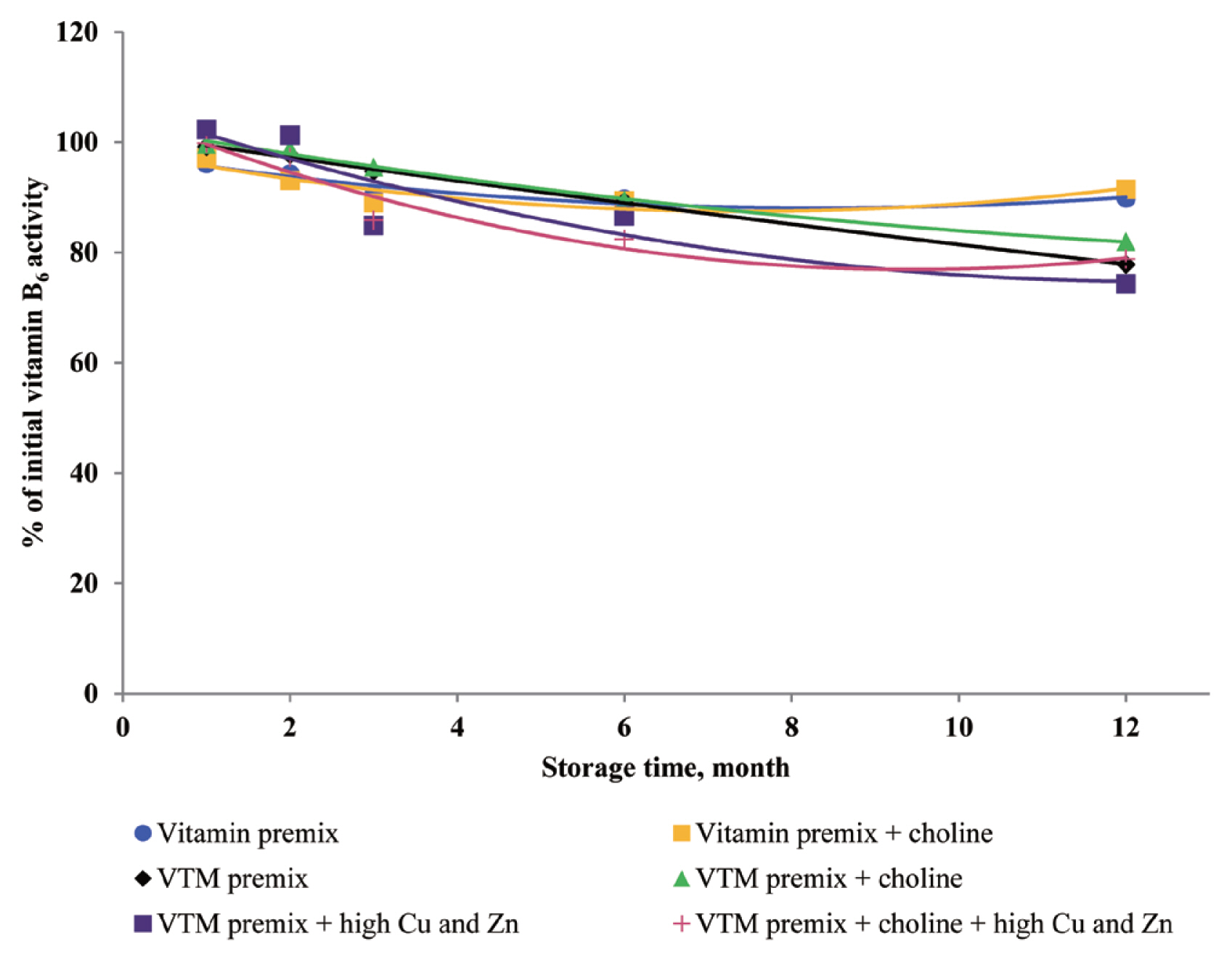
6 can lose bioactivity, particularly when minerals in the form of carbonates or oxides are present [
8,
11]. In the current study, the supplementation of high concentrations of Cu and Zn significantly reduced the concentrations of VB
6 in VTM premixes during storage. The loss of VB
6 activity after three months of storage at room temperature was 24% [
11], which was slightly higher than VB
6 loss in the present study. It may be that the degradation reaction of VB
6 was enhanced by metal ions in the previous study. Loss of VB
6 was lower when stored as vitamin premixes compared to VTM premixes, maybe the reason was that VTM premix contained more trace mineral. In VTM premixes, VB
6 can lose bioactivity, particularly when minerals in the form of carbonates or oxides are present [
8,
11]. We used pyridoxine hydrochloride in the present trial, which is a commercially available form. And pyridoxine hydrochloride is the main supplement used in feed, because it has good handling properties and stability. In addition, Coelho [
8] reported the loss of VB
6 after six months of storage in a vitamin premix was 17%, and 32% in a VTM premix. Although the retention rate of VB
6 in CoelhoŌĆÖs study [
8] was completely inconsistent with our results, our data also show that the stability of VB
6 is reduced in the premix containing inorganic trace minerals.
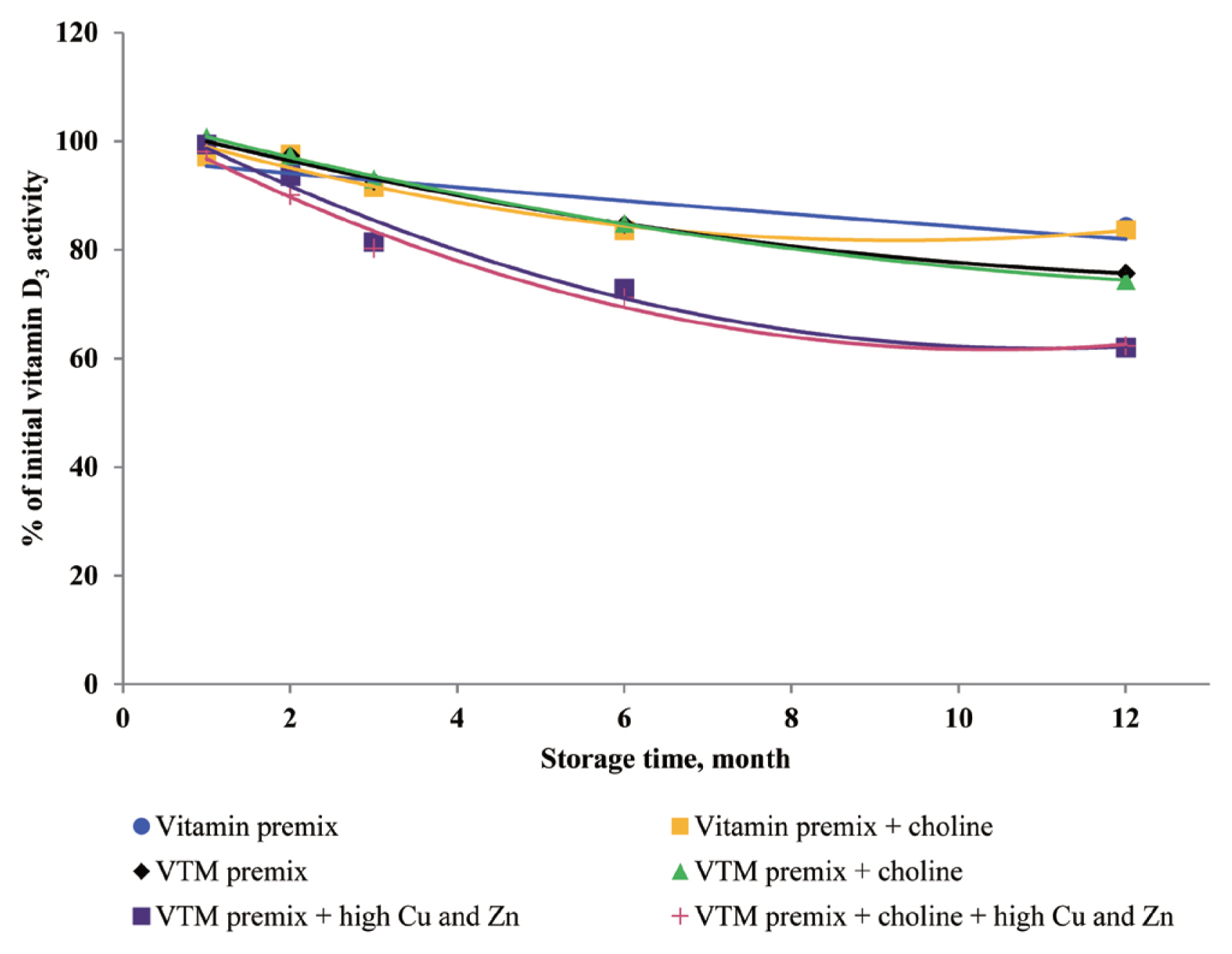
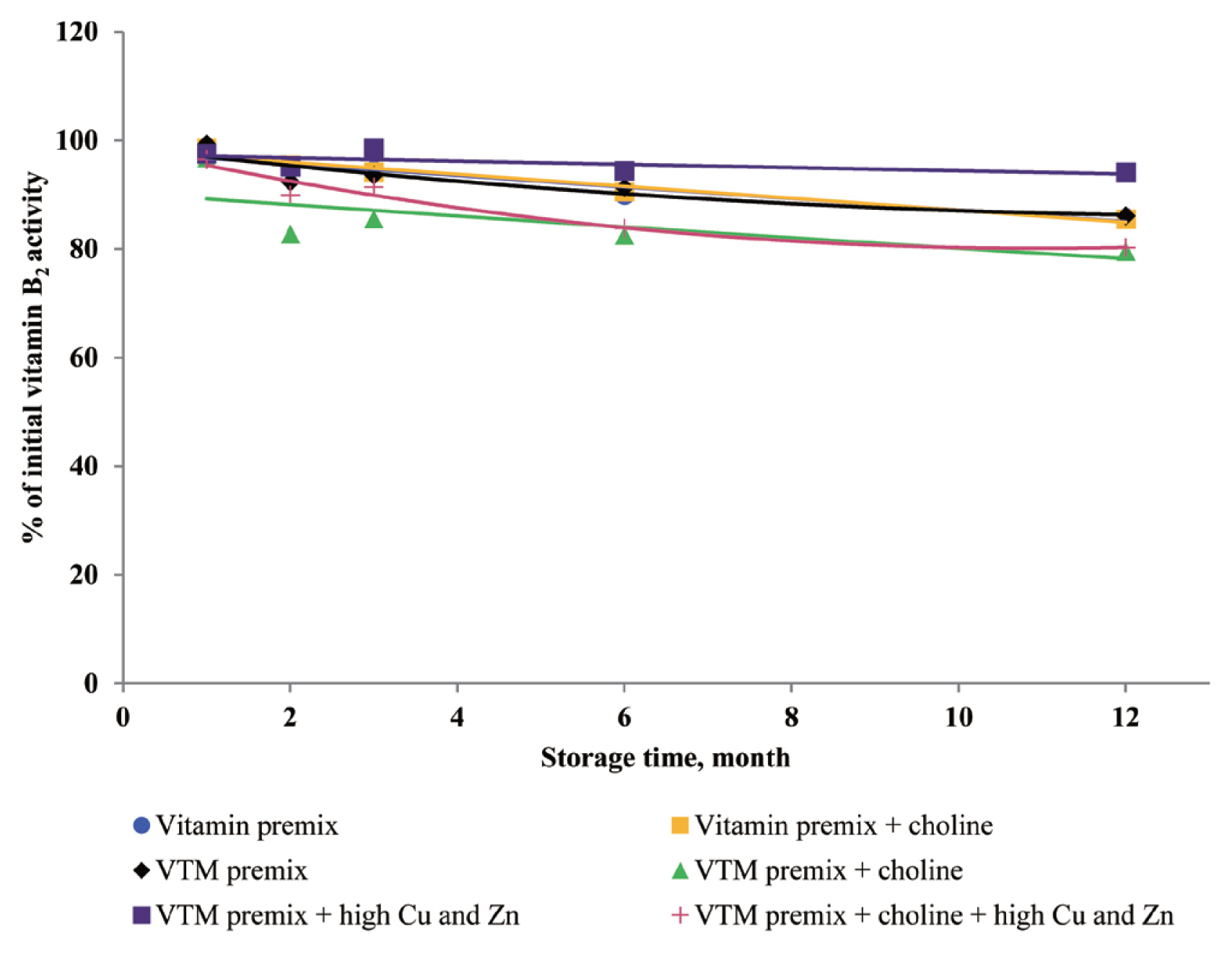
There are very limited stability data available for VD
3 and VB
2 in VTM premixes. The supplementation of high concentrations of Cu and Zn decreased the stability of VD
3 and VB
2 in VTM premixes in the present study. The VD
3 is a fundamentally unstable compound containing double bounds that can be altered by different stresses and prone to degradation due to oxidation [
3,
28]. The stability of VD to oxidation and its instability to trace minerals were also reported by previous studies; Mahmoodani et al [
28] demonstrated catalyzed isomerization of VD
3 is liable
via autoxidation to form a variety of oxidation products. Further, Zhuge and Klopfenstein [
19] reported that VB
2 was destroyed faster in the premix containing minerals and 54% of VB
2 had been destroyed after 27 weeks of storage. In the last case, the loss of VB
2 after six months of storage was 44% cited by Whitehead [
21]. The most well-characterized aspect of riboflavin reactivity is its sensitivity to light in an aerobic environment which may be one of the reasons for low VB
2 retention of previous studies. The rate of degradation can be promoted by complexation with some metal cations (e.g., Cu
2+ and Zn
2+) at the isoalloxazine moiety [
29]. Vitamin A, VD
3, VK
3, VB
1, VB
2, and VB
6 are relatively stable in vitamin premix, but degradation of vitamin was potentiated by the chemical reaction caused by the presence of Cu and Zn elements.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





