INTRODUCTION
MATERIALS AND METHODS
Ethical approval of animal use and location
Description and management of animals
Measurements and data collection
Experiment 1
Faecal sample analysis
Experiment 2
Animals: Six clinically healthy non-pregnant Nguni cows were randomly selected from animals used for experiment 1. The cows had a mean weight of 407 kg (range: 336 to 506 kg) and each cow was used as an experimental unit. Samples were collected between 08:30 and 11:30 h at ambient temperatures of not more than 30°C (Figure 1).
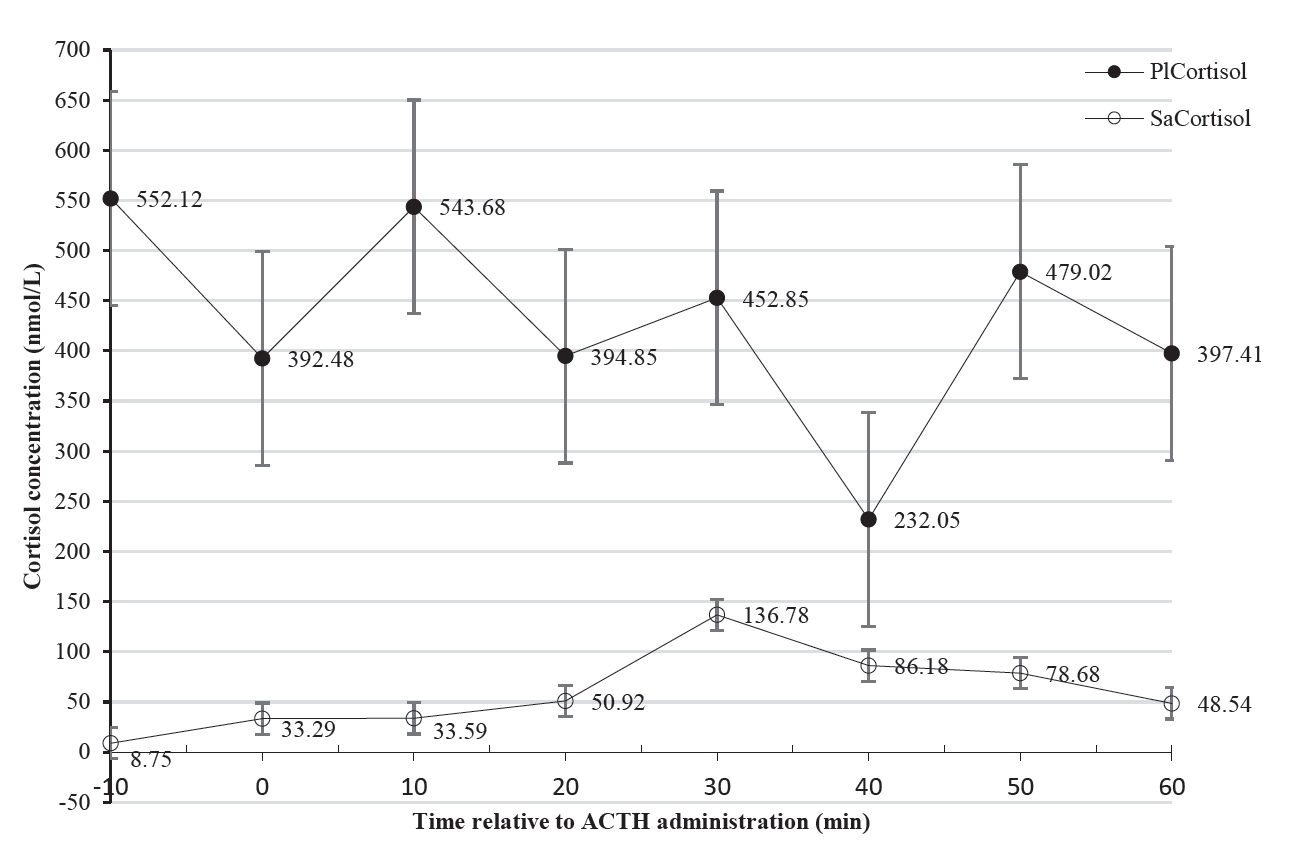
Adrenocorticotropic hormone administration: A standardised dose (1 μg/kg), of ACTH (Synacthen Depot, tetracosactide 1 mg/mL, Lot S1358, Novartis, South Africa) was administered once to each of the six cows at time 0 [27].
Extraction of blood samples: Blood samples (approximately 6 mL) were collected whilst the animals were restrained in a crush pen with the head in a head gate through jugular venepuncture into tubes containing SST gel. A nose grip was also used for stable restraint. Baseline samples were collected 10 min prior to ACTH administration. Thereafter, samples were drawn every 10 min for 1 h [28], to obtain a total of eight samples per animal. Samples were placed on wet ice (4°C) and then centrifuged for 10 min at 3,550×g (22°C). The serum was then transferred to red-topped tubes (4 mL) with a Clot Activator and stored at −20°C until analysis for cortisol.
Extraction of saliva samples: Saliva samples were collected immediately after blood sample collection using cotton based swabs (Salivette cortisol; Sarstedt, Nümbrecht-Rommelsdorf, Germany), which provided a method for easy and safe collection. Cotton balls were also used to collect drooling saliva to maximise the amount of saliva collected. The cotton swabs were inserted at an angle of the lips into the mouths of the cows with the help of a nose grip until well soaked [29]. Each swab and the corresponding cotton ball were then placed in the salivette tube which was placed on ice (4°C). The samples were centrifuged at 1,000×g for 2 min at 20°C. The swabs and cotton balls were removed together with the inner tube of the salivettes. The saliva which collected into the outer tubes was immediately stored at −20°C until analysis. Salivary cortisol was determined by competitive enzyme-linked immunosorbent assay (ELISA).
Cortisol analysis in plasma and saliva: Blood plasma samples were defrosted and vortexed (Vortex Genie-2, Scientific Industries Inc, New York, USA) at room temperature (24°C). Quantification of plasma cortisol was then done by ELISA using a Cortisol ELISA kit (IBL International, GmBH, Hamburg, Germany, RE52611) as described by Olbrich and Dittmar [30].






 ) and minimum (
) and minimum (
 ) environmental temperatures during the five sampling days for Experiment 1. The temperatures increased then decreased as indicated by the patterns of graphs and error bars represent standard error of means.
) environmental temperatures during the five sampling days for Experiment 1. The temperatures increased then decreased as indicated by the patterns of graphs and error bars represent standard error of means.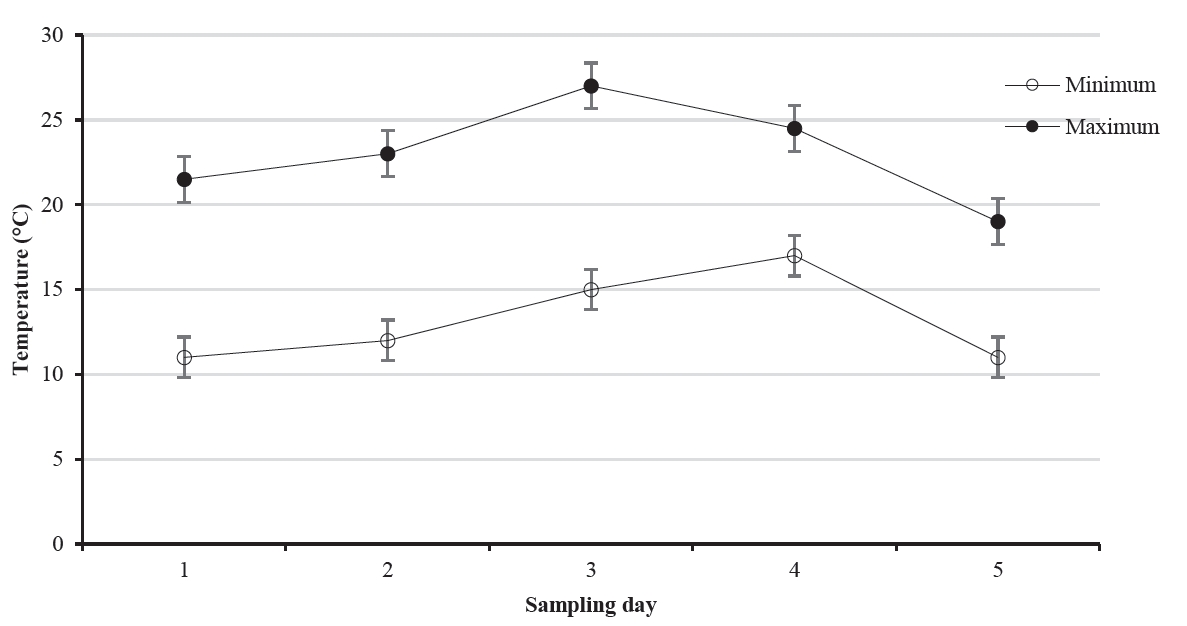
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print







