 |
 |
| Anim Biosci > Volume 37(6); 2024 > Article |
|
Abstract
Objective
R-loops are DNA:RNA triplex hybrids, and their metabolism is tightly regulated by transcriptional regulation, DNA damage response, and chromatin structure dynamics. R-loop homeostasis is dynamically regulated and closely associated with gene transcription in mouse zygotes. However, the factors responsible for regulating these dynamic changes in the R-loops of fertilized mouse eggs have not yet been investigated. This study examined the functions of candidate factors that interact with R-loops during zygotic gene activation.
Methods
In this study, we used publicly available next-generation sequencing datasets, including low-input ribosome profiling analysis and polymerase II chromatin immunoprecipitation-sequencing (ChIP-seq), to identify potential regulators of R-loop dynamics in zygotes. These datasets were downloaded, reanalyzed, and compared with mass spectrometry data to identify candidate factors involved in regulating R-loop dynamics. To validate the functions of these candidate factors, we treated mouse zygotes with chemical inhibitors using in vitro fertilization. Immunofluorescence with an anti-R-loop antibody was then performed to quantify changes in R-loop metabolism.
Results
We identified DEAD-box-5 (DDX5) and histone deacetylase-2 (HDAC2) as candidates that potentially regulate R-loop metabolism in oocytes, zygotes and two-cell embryos based on change of their gene translation. Our analysis revealed that the DDX5 inhibition of activity led to decreased R-loop accumulation in pronuclei, indicating its involvement in regulating R-loop dynamics. However, the inhibition of histone deacetylase-2 activity did not significantly affect R-loop levels in pronuclei.
Conclusion
These findings suggest that dynamic changes in R-loops during mouse zygote development are likely regulated by RNA helicases, particularly DDX5, in conjunction with transcriptional processes. Our study provides compelling evidence for the involvement of these factors in regulating R-loop dynamics during early embryonic development.
Remodeling of the chromatin architecture has been identified as a crucial driving force for zygotic gene expression in various species [1,2]. A recent study highlighted the role of nucleosome-free regions and histone acetylation, particularly H3K9ac and H3K27ac, in the activation of genes involved in zygotic gene activation (ZGA), independent of DNA replication or transcriptional elongation [3]. These nucleosome-free regions are believed to be established through the binding of pioneer transcription factors that are necessary for ZGA. Additionally, the incorporation of RNA polymerase II (RNAPII) into the zygotic genome is associated with ZGA in mice [4,5]. Liu et al [5] demonstrated that RNAPII loading occurs in both activated and silenced genes during major ZGA and displays weak elongation activity during minor ZGA.
RNAPII occupancy is observed in both paused and productive elongation states during gene transcription [6–8]. Generally, RNAPII pausing inhibits transcription initiation and affects R-loop formation [9–12]. R-loops are DNA:RNA hybrids that are naturally synthesized in vivo and localized to the genome in a sequence-specific manner. R-loops play important regulatory roles in various cellular processes, including DNA replication, transcription, immunoglobulin G (IgG) class switch recombination, and DNA [13–17]. Although R-loops are potentially harmful structures that can lead to genomic instability and DNA damage, they are unavoidably formed during many cellular processes [18]. Their presence and proper regulation are critical for maintaining cellular homeostasis and preventing DNA damage [19,20]. Recent studies have identified R-loop-interacting proteins involved in replication, transcriptional regulation, alternative splicing, and DNA repair using mass spectrometry combined with immunoprecipitation (IP) with R-loop-specific S9.6 antibodies [21]. These proteins include RNA helicases, RNA processing factors, and chromatin remodelers [21,22]. Among these, a subset of RNA helicases belonging to the DEAH/RHA (DHX) and DEAD-box (DDX) families, particularly DDX5 and DHX9, have been identified as critical regulators of R-loop biogenesis and resolution [23–27]. Although the term “RNA helicases” implies unwinding RNA species, only a subset of them exhibits processive helicase activity [28–30]. Certain RNA helicases, including DDX1, DDX5, DDX17, DDX20, DDX21, and DHX9, have been implicated as coactivators or corepressors in transcriptional regulation [31]. In the case of DDX5, arginine methylation of its RGG/RG domain by protein arginine methyltransferase 5 (PRMT5) regulates R-loop levels, particularly at transcription termination sites [23,32]. The RGG/RG domain is required for interaction with RNAPII subunit A (POLR2A) and 5′-3′ exoribonuclease 2 (XRN2), facilitating the release of RNAPII and preventing R-loop formation [23,33–35].
Studies have demonstrated that the function of DDX5 in regulating R-loop dynamics is context-dependent [23,32,33, 36–38]. DDX5 promotes R-loop resolution under normoxia but promotes R-loop biogenesis under hypoxia [38]. Therefore, the precise role of DDX5 requires further investigation in different cell types, including preimplantation embryos. Although many studies have attempted to understand the biogenesis and resolution of R-loops and have demonstrated their diverse molecular functions in vivo and in vitro, very few studies have focused on the role of R-loops in early preimplantation embryos, particularly their involvement in the regulation of zygotic gene expression. In this study, we examined candidate factors that interact with R-loops using chemical inhibitors and found that RNA helicase DDX5 plays a critical role in R-loop metabolism in mouse zygotes.
Female ICR mice aged 6 to 8 weeks and male ICR mice aged 8 to 10 weeks were obtained from Orient Bio Co., Ltd. (Seoul, Korea). All procedures involving mice were conducted in accordance with guidelines approved by the Institutional Animal Care and Use Committee of Konkuk University (IACUC approval number: KU22181). The mice were housed in a controlled environment at 22°C±1°C with a 12-h light-dark cycle.
To induce superovulation in female mice, intraperitoneal injections of pregnant mare serum gonadotropin (5 IU) were administered, followed by human chorionic gonadotropin (hCG; 5 IU) 48 h later. Fifteen hours after hCG injection, cumulus-oocyte complexes (COC) were collected from the oviduct and transferred to a modified human tubal fluid (mHTF) medium supplemented with 0.625 mM glutathione (GSH; Sigma-Aldrich, St. Louis, MO, USA). Spermatozoa were collected from the caudal epididymis of male mice and incubated in mHTF medium containing 0.4 mM methyl-β-cyclodextrin (MBCD)-polyvinyl alcohol (PVA) for at least an hour to induce sperm capacitation. Capacitated sperm was then added to the GSH-mHTF medium containing COCs. Two hours after insemination, the fertilized embryos were washed multiple times and cultured in EmbryoMax KSOM Mouse Embryo media (Merck Millipore, Burlington, MA, USA) at 37°C under 5% CO2.
Each inhibitor was added and incubated for 3 days, and dimethyl sulfoxide (DMSO; Sigma-Aldrich, USA) was used as a vehicle for inhibitors in the control group. Embryos were transferred to the KSOM medium containing either DMSO or a specific inhibitor at 4 h post-insemination (hpi) and cultured for 8 h (12 hpi). The following inhibitors were used in the present study: 5 or 100 nM trichostatin A (TSA; Sigma-Aldrich, USA) for inhibition of histone deacetylase 2 (HDAC2), 20 nM supinoxin (RX-5902; MedChemExpress, Monmouth Junction, NJ, USA) for DDX5 inhibition, and 100 μM 5,6-dichlorobenzimidazole 1-β-D-ribofuranoside (DRB; Sigma-Aldrich, USA) and 1 μM triptolide (TRP; Sigma-Aldrich, USA) for inhibition of transcription. After inhibitor treatment, the embryos were fixed with 4% paraformaldehyde in a solution containing 0.01% PVA in phosphate-buffered saline (PBS) for 15 min. They were then washed thrice with a washing buffer (0.05% Tween 20-0.01% PVA-PBS) for 10 min each. The embryos were permeabilized with 0.5% Triton X-100-PVA in PBS for 20 min, followed by three washes. The embryos were treated with 4 N HCl for 15 min to denature the DNA and then neutralized with 100 mM Tris-HCl (pH 8.5) for 20 min. Subsequently, the embryos were blocked by incubation with 5% bovine serum albumin (BSA)-PVA/PBS for 2 h. Primary antibodies, including anti-S9.6 antibodies (1:200, MABE1095; Sigma-Aldrich, USA) and anti-phospho-histone H2A.X (1:300, 2577; Cell Signaling Technology, Danvers, MA, USA), were diluted in 1% BSA-PVA/PBS and incubated with the embryos at 4°C overnight. After three washes, the embryos were incubated with secondary antibodies, including donkey anti-mouse IgG Alexa Fluor 488 conjugate (1:400; Life Technologies, Carlsbad, CA, USA) and donkey anti-rabbit IgG Alexa Fluor 546 conjugate (1:400; Life Technologies, USA), for 1 h in the dark. DNA was counterstained with 2 μM To-PRO3 Iodine (Thermo Fisher Scientific, Waltham, MA, USA) for 20 min. The stained embryos were washed several times and mounted on slides using a small drop of VECTASHIELD Antifade Mounting Medium (Vector Laboratories, Burlingame, CA, USA).
A publicly available bulk RNA-seq and low-input ribosome profiling (called LiRibo-seq) dataset [39] was used to examine the patterns of protein synthesis in mouse oocyte, 1-cell and 2-cell embryos. The dataset (GSE169632) was downloaded and reanalyzed, and the fragments per kilobase of transcripts per million mapped reads (FPKM) values of actively translated mRNAs were determined using DESeq2 tools. RNAPII ChIP-seq dataset (GSE135457) analyzed in oocyte, 1-cell and 2-cell embryos were used to identify the actively transcribed genes [5]. From the intersection of two independent IP-mass spectrometry (IP-MS) studies [21,22], a set of 205 proteins known to interact with R-loops was selected for further analysis based on their synthesis levels in the LiRibo-seq dataset.
All images were captured using a laser confocal microscope (LSM 8000; Zeiss, Oberkochen, Germany). For 1-cell stage embryos, the middle optical section of Z-stack images containing the maternal and paternal pronuclei was selected for fluorescence intensity quantification. The fluorescence intensity of the S9.6 signal within the pronuclei was measured using the ImageJ software (National Institute of Health, Bethesda, MD, USA). Quantification was performed using the following approach: the total intensity of the pronucleus was calculated by multiplying the mean intensity of the pronucleus region by the area of the pronucleus and then subtracting the product of the mean intensity of the cytoplasmic region by the area of the pronucleus.
Statistical analyses were performed using Prism software (GraphPad Inc., Boston, MA, USA). A two-tailed unpaired t-test with Welch’s correction was used to compare the two groups. One-way analysis of variance followed by Tukey’s test was performed for comparisons among more than three groups. The results are presented as mean±standard error of the mean or mean±standard deviation. Statistical significance is indicated as follows: * p<0.05, ** p<0.01, *** p<0.001, NS, not significant.
To identify potential regulators of R-loop homeostasis in oocyte and early embryos, we analyzed publicly available LiRibo-seq (GSE169632) [39] and RNAPII ChIP-seq datasets (GSE135457) generated from mouse oocyte, 1-cell and 2-cell embryos [5]. From these datasets, 205 proteins that were previously identified as R-loop-interacting proteins [21,22] were selected (Figure 1A; Table 1), and their dynamic changes of gene translation from oocytes to 2-cell stage embryos were investigated. As depicted in Figure 1A, the levels of transcripts undergoing translation differed significantly from those detected in bulk RNA-seq, indicating a specific regulation of translation during early development. Hierarchical clustering analysis of the 205 proteins revealed four distinct clusters: cluster 1 (C1, 120 genes) displayed active translation at the 2-cell stage, C2 (39 genes) exhibited active translation at the oocyte stage, C3 (12 genes) showed active translation at both the oocyte and 1-cell stages, and C4 (24 genes) displayed active translation at the 1-cell stage. Additionally, enrichment of RNAPII at the transcription start sites (TSSs) was observed for most of the 205 genes, yet the RNAPII enrichment was greatly diminished upon DRB treatment (Figure 1A). Based on the translation profiles of these genes, we selected two candidate proteins known for their roles in RNA unwinding, DNA damage repair, and histone modification from the gene clusters (Figure 1B) and employed chemical inhibitors to investigate their functions in the regulation of R-loop metabolism during the ZGA period. The inhibitors used in this study were TSA, which inhibits HDAC2 (a gene from cluster C2), and supinoxin, which inhibits DDX5 (a gene from cluster C4). Interestingly, other class I HDAC genes displayed a distinct pattern of translation in the stages (Figure 1B). Gene ontology (GO) term analysis indicated that the genes within these four clusters were mainly associated with RNA processing and splicing, suggesting their involvement in RNA biology (Figure 1C).
In our previous study, we established a correlation between zygotic R-loop dynamics and gene transcription. To validate the finding, additional experiments were performed using DRB (Figure 2A, 2B), a transcription elongation inhibitor, and TRP (Figure 2C, 2D), a transcription initiation inhibitor. After in vitro fertilization, 1-cell embryos were treated with either TRP or DRB. Consistently, the transcriptional inhibition with either inhibitor significantly reduced R-loop formation in both maternal (M) and paternal (P) pronuclei (PN) (Figure 2B, 2D).
Given that the level of translated HDAC2 was high in and diminished in 1-cell and 2-cell embryos (Figure 1A), and HDAC2 function has been implicated in the ZGA [40], 1-cell embryos were treated with TSA to determine the role of HDAC2 in regulating R-loop formation during minor ZGA. The TSA treatment had little or no effect on the R-loop levels in both the maternal and paternal pronuclei of 1-cell embryos (Figure 3A, 3B). We also found that the levels of histone H3K27ac were slightly increased by both low and high concentrations of TSA (Figure 3A, 3C).
Next, to investigate the function of RNA helicases in R-loop formation during minor ZGA of mouse zygotes, 1-cell stage embryos were treated with supinoxin (RX5902), an RNA helicase DDX5 inhibitor. The DDX5 inhibition [23] led to a notable reduction in the number of R-loop foci observed in 1-cell embryos (Figure 4A, 4B), whereas its inhibition elevated γH2AX level in both maternal and paternal pronuclei (Figure 4A, 4C). These results provide compelling evidence that RNA helicases, specifically DDX5, play critical roles in the regulation of R-loop metabolism in 1-cell embryos during minor ZGA.
In this study, we identified candidate factors that may be involved in the production and resolution of R-loops and validated the function of selected factors using chemical inhibitors during the development of early preimplantation embryos. Two hundred and five candidate factors that potentially regulate R-loop formation were identified through DNA:RNA hybrid MS combined with IP studies using the S9.6 antibody and hybrid binding domain of RNase H1 (Figure 1A) [21,22]. In particular, the translation levels of these proteins exhibited different patterns as determined by low-input ribosome profiling analysis (Figure 1B). Proteins were divided into four clusters based on the translation status from MII oocytes to the 2-cell embryo stage; HDAC2 and DDX5 were selected from clusters 2 and 4, respectively. Chemical inhibitors targeting these candidates were used to investigate their effects on the R-loop biogenesis in minor ZGA.
A recent study showed that both HDAC1 and HDAC2 are required to regulate H3K27ac levels and ZGA in bovines [40]. Furthermore, deletion of both genes in growing oocytes leads to follicle development arrest at the secondary follicle stage [41]. To examine the effect of HDAC2 on R-loop formation in zygotes, R-loop levels were analyzed by immunofluorescence using the S9.6 antibody with and without treatment with TSA (an HDAC inhibitor). Although a slight increase in histone H3K27ac level was observed in the low TSA-treated group, this increase was not statistically significant. Notably, R-loop levels were not affected by the inhibition of HDAC2, regardless of the TSA concentration used. During ZGA, HDAC plays a crucial role in the regulation of H3K27ac, a marker of active promoters. The levels of H3K27ac dynamically change during this stage and increase in zygotes [42,43]. Our analysis suggests that aberrant H3K27ac levels resulting from HDAC inhibition do not affect R-loop formation in 1-cell embryos.
Timely and safe development of life during the preimplantation stages relies on the shift of transcripts from the oocyte to newly synthesized ones from the zygotic genome, a process known as maternal-to-zygotic transition (MZT) that occurs during ZGA [4,44,45]. Despite extensive research, the precise mechanism of MZT action remains unclear. The MZT is characterized by the transition of translational transcripts from maternal to zygotic origins [45]. The proper occurrence of minor and major ZGA is essential to ensure MZT. Pharmacological interference with transcription results in aberrant gene expression and developmental arrest [4]. Additionally, minor ZGA is closely associated with histone H3K4 methylation, as loss of H3K4 methylation impairs minor ZGA in the paternal pronucleus [46].
Following fertilization, a punctuated pattern of R-loop distribution throughout the genome has been observed in the zygotic pronuclei [47]. Although R-loop homeostasis is associated with both DNA replication and transcription, no study has elucidated the factors known to regulate R-loop metabolism in the minor ZGA of mouse zygotes.
Recent studies have provided evidence that DDX5 depletion in cells leads to the accumulation of R-loops near TSSs [33]. Furthermore, arginine methylation of the RGG/RG motif of DDX5 by PRMT5 and XRN resolved R-loops in U2OS cells [33]. Inhibition of DDX5 during the 1-cell stage resulted in a significant decrease in R-loop levels in both maternal and paternal pronuclei (Figure 4A, 4C), accompanied by an increase in γH2AX levels, a marker of DNA damage. Studies have demonstrated that DDX5 inhibition increases γH2AX levels, indicating its role in DNA damage repair [36,37]. Downregulation of DDX5 in embryos impedes normal pronucleus formation and development [48]. Therefore, defects in pronuclear development caused by DDX5 inhibition may lead to increased DNA damage and suppressed R-loop formation. This finding is consistent with those of our previous study, which showed that zygotes failed to promote R-loop formation during DNA damage repair.
The decreased S9.6 intensity observed in DDX5-inhibited zygotes can be explained by the reduced transcriptional activity of mature oocytes, which is maintained until ZGA occurs. Maternal RNA processing plays a crucial role during early development, particularly in the 1-cell stage embryo. A large proportion of proteins that interact with R-loops are involved in RNA splicing, processing, and ribosome biogenesis (Figure 1C). This suggests that DDX5 helicases unwind mRNA secondary structures and interact with R-loops for normal pronuclear development in the 1-cell stage embryo. Therefore, the decreased R-loop level observed in DDX5-inhibited zygotes may be attributed to reduced helicase activity, which hinders RNA strand displacement from the non-template DNA, thereby inhibiting R-loop formation.
Our analysis of the LiRibo-seq data revealed that GO terms enriched across all clusters were predominantly associated with RNA processing, highlighting the important role of RNA helicases and their relationship with R-loops in early embryonic development. As shown in (Figure 1C), RNA helicases, which play a role in unwinding RNA molecules, have been implicated in various RNA processing events, including transcription, RNA splicing, and RNA transport, by modulating RNA-RNA, RNA-DNA, and RNA-protein interactions. The DEAH/RHA (DHX) and DEAD-box (DDX) families of RNA helicases have been shown to regulate R-loop biogenesis and influence genome stability and DNA damage repair.
Notably, the function of DDX5 may vary depending on the cellular context. Although DDX5 has generally been implicated in R-loop resolution [23,32,33,36–38], recent studies have shown its involvement in R-loop formation under hypoxic conditions [38]. Therefore, it would be interesting to investigate how the function of DDX5 in zygotes differs from that in normal somatic cells. Additionally, the identification and functional validation of other factors that regulate DDX5 is crucial for a comprehensive understanding of its role in R-loop regulation.
Notes
Figure 1
Identification of candidate factors that potentially regulate R-loop metabolism during zygotic genome activation. (A) Two hundreds five proteins were selected from two independent proteomics studies that identified R-loop-interacting proteins [21,22]. Publicly available bulk RNA-seq (GSE169632), RNAPII ChIP-seq (GSE135457), and low-input ribosome profiling (LiRibo-seq, GSE169632) datasets were analyzed. Heatmaps showing abundance of RNAs, enrichment of RNAPII at TSS ±3,000 bps, and differentially translating genes from zygote to 2-cell stage embryos. (B) Heatmaps showing translating RNA levels of DEAD-box-5 (DDX5) and histone deacetylase-2 (HDAC2). (C) Analysis of gene ontology (GO) of the differentially translating genes in cluster 1 (120 genes), cluster 2 (39 genes), cluster 3 (12 genes), and cluster 4 (24 genes).
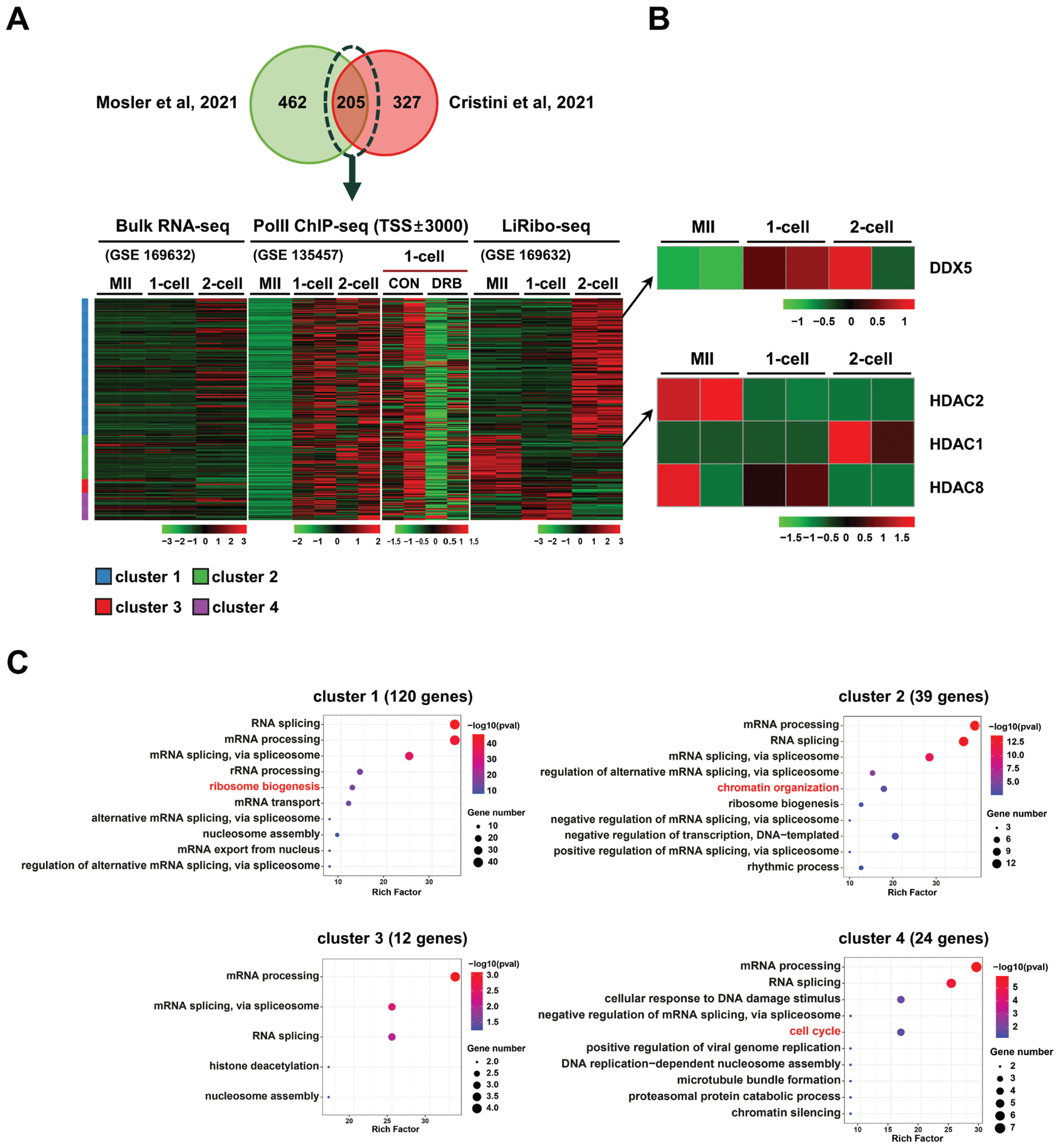
Figure 2
Dynamics of R-loop metabolism in response to inhibition of gene transcription. (A) Representative immunofluorescent images using anti-S9.6 (green) and anti-gH2A.X (red) antibodies after treatment with 100 μM 5,6-dichlorobenzimidazole 1-β-D-ribofuranoside (DRB), and quantification of their intensities (B). TO-PRO3 was used for the staining of nuclear DNAs. (C) Representative immunofluorescent images using anti-S9.6 (green) and anti-gH2A.X (red) antibodies after treatment with 1 μM triptolide (TRP) and quantification of their intensities (D). TO-PRO3 was used for the staining of DNAs. Scale bars, 5 μm. M.PN, maternal pronucleus. P.PN, paternal pronucleus. CON, Control. Graphs in (B) and (D) represent the mean and standard error mean. *** p<0.001.
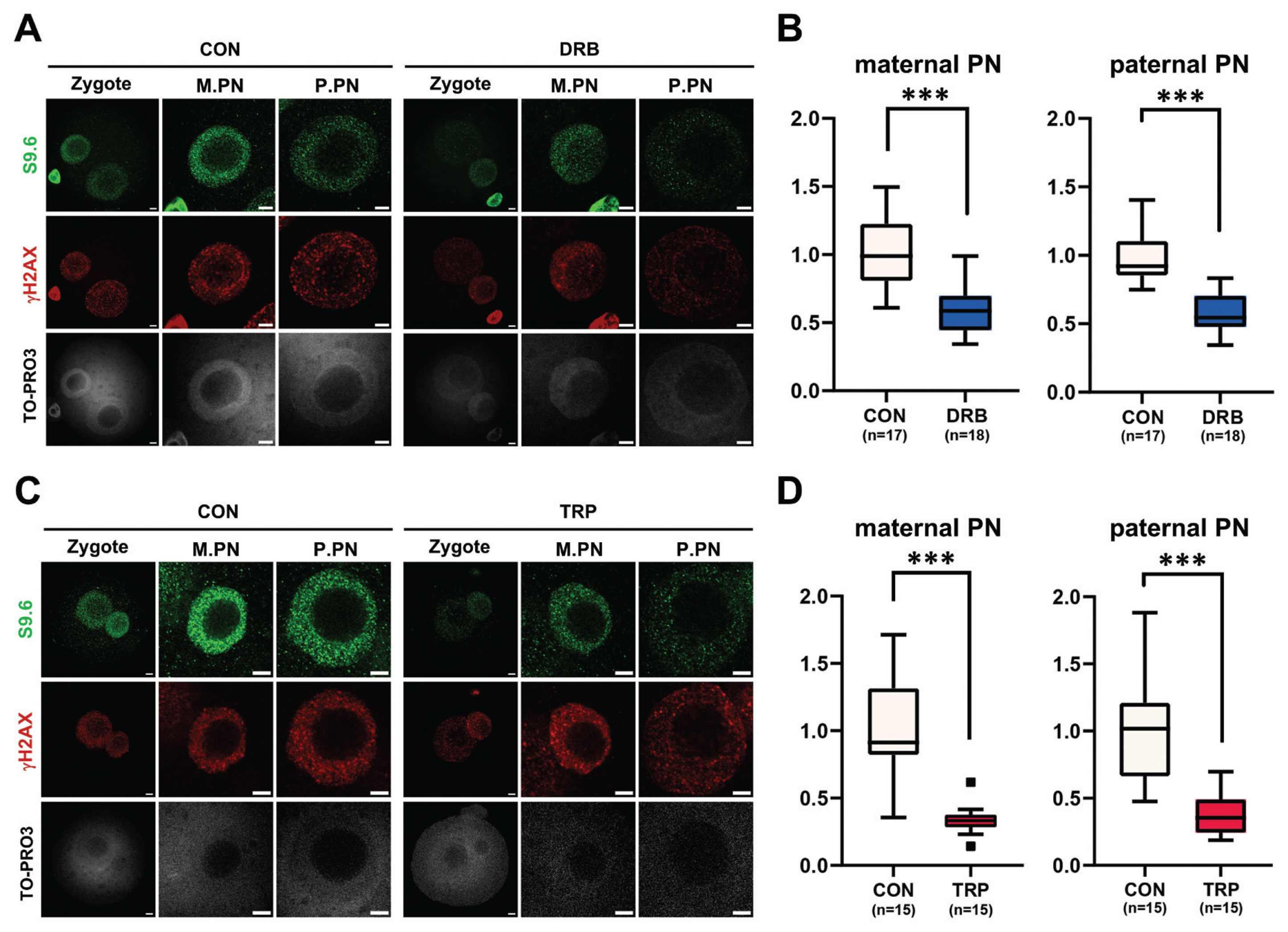
Figure 3
Effect of histone deacetylase-2 (HDAC2) inhibition on R-loop metabolism. (A) Representative immunofluorescence using anti-S9.6 (green) and anti-H3K27ac (red) antibodies after treatment with different concentrations (5 or 100 nM) of trichostatin A (TSA). (B, C) Quantification of signal intensities of immunofluorescence of S9.6 (B) and H3K27ac (C). M.PN, maternal pronucleus. P.PN, paternal pronucleus. Scale bars, 5 μm. CON, Control. Graphs represent the mean and standard error mean.

Figure 4
Effect of DEAD-box-5 (DDX5) inhibition on R-loop metabolism. (A) Representative immunofluorescence using anti-S9.6 (green) and anti-gH2A.X (red) antibodies after treatment with 20 nM supinoxin. (B, C) Quantification of signal intensities of immunofluorescence of S9.6 (B) and γH2AX (C). M.PN, maternal pronucleus. P.PN, paternal pronucleus. Scale bars, 5 μm. CON, control. Graphs represent the mean and standard error mean. * p<0.05. *** p<0.001.
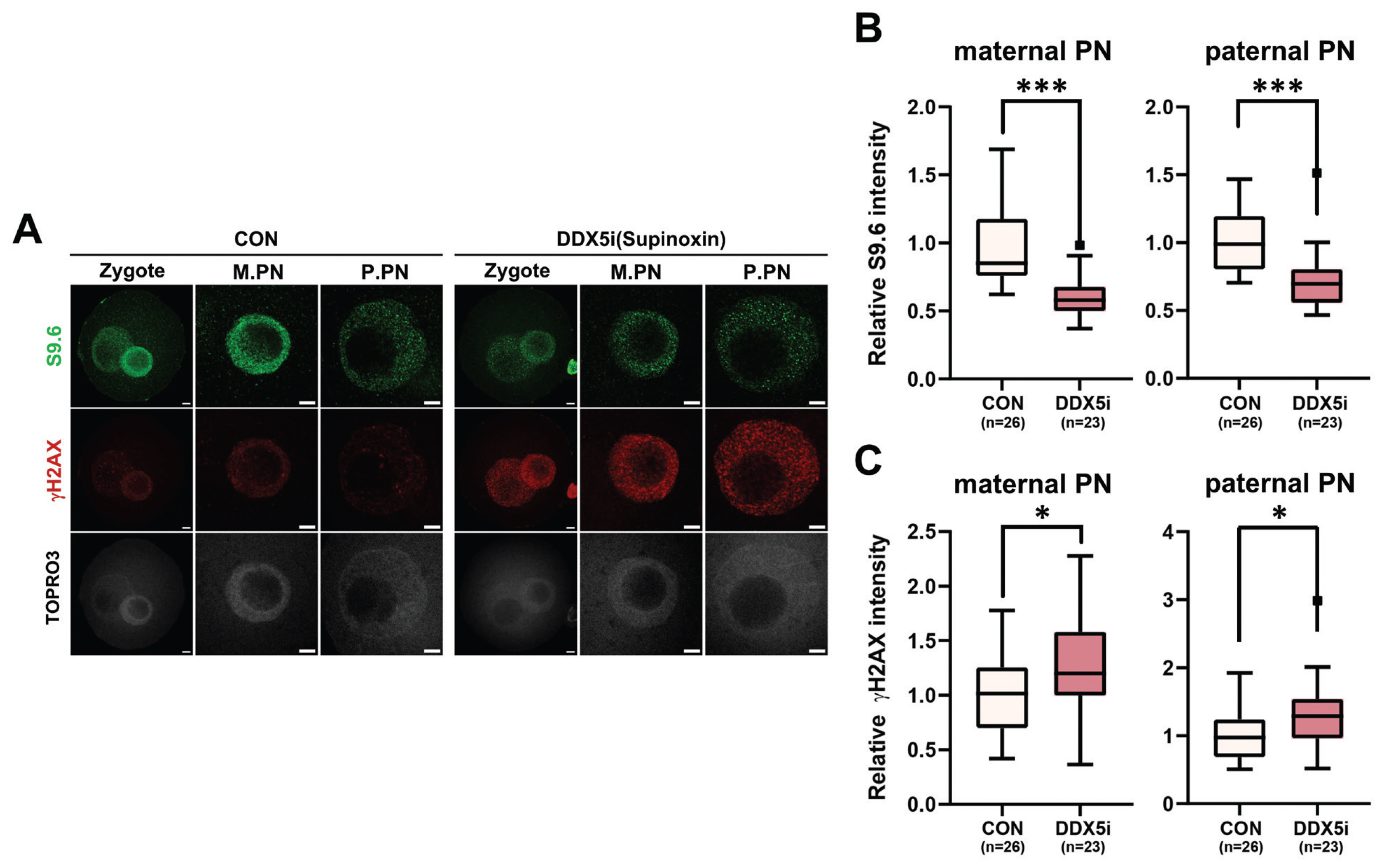
Table 1
List of candidate genes regulating R-loop metabolism
REFERENCES
1. Ing-Simmons E, Rigau M, Vaquerizas JM. Emerging mechanisms and dynamics of three-dimensional genome organisation at zygotic genome activation. Curr Opin Cell Biol 2022; 74:37–46.
https://doi.org/10.1016/j.ceb.2021.12.004


2. Eckersley-Maslin MA, Alda-Catalinas C, Reik W. Dynamics of the epigenetic landscape during the maternal-to-zygotic transition. Nat Rev Mol Cell Biol 2018; 19:436–50.
https://doi.org/10.1038/s41580-018-0008-z


3. Wang C, Chen C, Liu X, et al. Dynamic nucleosome organization after fertilization reveals regulatory factors for mouse zygotic genome activation. Cell Res 2022; 32:801–13.
https://doi.org/10.1038/s41422-022-00652-8



4. Abe KI, Funaya S, Tsukioka D, et al. Minor zygotic gene activation is essential for mouse preimplantation development. Proc Natl Acad Sci USA 2018; 115:E6780–8.
https://doi.org/10.1073/pnas.1804309115



5. Liu B, Xu Q, Wang Q, et al. The landscape of RNA Pol II binding reveals a stepwise transition during ZGA. Nature 2020; 587:139–44.
https://doi.org/10.1038/s41586-020-2847-y


6. Core L, Adelman K. Promoter-proximal pausing of RNA polymerase II: a nexus of gene regulation. Genes Dev 2019; 33:960–82.
https://doi.org/10.1101/gad.325142.119



7. Abuhashem A, Garg V, Hadjantonakis AK. RNA polymerase II pausing in development: orchestrating transcription. Open Biol 2022; 12:210220
https://doi.org/10.1098/rsob.210220



8. Price DH. Transient pausing by RNA polymerase II. Proc Natl Acad Sci USA 2018; 115:4810–2.
https://doi.org/10.1073/pnas.1805129115



9. Shao W, Zeitlinger J. Paused RNA polymerase II inhibits new transcriptional initiation. Nat Genet 2017; 49:1045–51.
https://doi.org/10.1038/ng.3867


10. Castillo-Guzman D, Chédin F. Defining R-loop classes and their contributions to genome instability. DNA Repair (Amst) 2021; 106:103182
https://doi.org/10.1016/j.dnarep.2021.103182



11. Zhang X, Chiang HC, Wang Y, et al. Attenuation of RNA polymerase II pausing mitigates BRCA1-associated R-loop accumulation and tumorigenesis. Nat Commun 2017; 8:15908
https://doi.org/10.1038/ncomms15908



12. Zardoni L, Nardini E, Brambati A, et al. Elongating RNA polymerase II and RNA:DNA hybrids hinder fork progression and gene expression at sites of head-on replication-transcription collisions. Nucleic Acids Res 2021; 49:12769–84.
https://doi.org/10.1093/nar/gkab1146



13. Gan W, Guan Z, Liu J, et al. R-loop-mediated genomic instability is caused by impairment of replication fork progression. Genes Dev 2011; 25:2041–56.
https://doi.org/10.1101/gad.17010011



14. Pohjoismäki JL, Holmes JB, Wood SR, et al. Mammalian mitochondrial DNA replication intermediates are essentially duplex but contain extensive tracts of RNA/DNA hybrid. J Mol Biol 2010; 397:1144–55.
https://doi.org/10.1016/j.jmb.2010.02.029



15. Huertas P, Aguilera A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol Cell 2003; 12:711–21.
https://doi.org/10.1016/j.molcel.2003.08.010


16. Yu K, Chedin F, Hsieh CL, Wilson TE, Lieber MR. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol 2003; 4:442–51.
https://doi.org/10.1038/ni919


17. Ohle C, Tesorero R, Schermann G, Dobrev N, Sinning I, Fischer T. Transient RNA-DNA hybrids are required for efficient double-strand break repair. Cell 2016; 167:1001–13.
https://doi.org/10.1016/j.cell.2016.10.001


18. Aguilera A, García-Muse T. R loops: from transcription byproducts to threats to genome stability. Mol Cell 2012; 46:115–24.
https://doi.org/10.1016/j.molcel.2012.04.009


19. Brickner JR, Garzon JL, Cimprich KA. Walking a tightrope: the complex balancing act of R-loops in genome stability. Mol Cell 2022; 82:2267–97.
https://doi.org/10.1016/j.molcel.2022.04.014



20. Petermann E, Lan L, Zou L. Sources, resolution and physiological relevance of R-loops and RNA-DNA hybrids. Nat Rev Mol Cell Biol 2022; 23:521–40.
https://doi.org/10.1038/s41580-022-00474-x


21. Cristini A, Groh M, Kristiansen MS, Gromak N. RNA/DNA hybrid interactome identifies DXH9 as a molecular player in transcriptional termination and R-loop-associated DNA damage. Cell Rep 2018; 23:1891–905.
https://doi.org/10.1016/j.celrep.2018.04.025



22. Mosler T, Conte F, Longo GMC, et al. R-loop proximity proteomics identifies a role of DDX41 in transcription-associated genomic instability. Nat Commun 2021; 12:7314
https://doi.org/10.1038/s41467-021-27530-y



23. Mersaoui SY, Yu Z, Coulombe Y, et al. Arginine methylation of the DDX5 helicase RGG/RG motif by PRMT5 regulates resolution of RNA:DNA hybrids. EMBO J 2019; 38:e100986
https://doi.org/10.15252/embj.2018100986



24. Saha S, Yang X, Huang SN, et al. Resolution of R-loops by topoisomerase III-beta (TOP3B) in coordination with the DEAD-box helicase DDX5. Cell Rep 2022; 40:111067
https://doi.org/10.1016/j.celrep.2022.111067



25. Dou P, Li Y, Sun H, et al. C1orf109L binding DHX9 promotes DNA damage depended on the R-loop accumulation and enhances camptothecin chemosensitivity. Cell Prolif 2020; 53:e12875
https://doi.org/10.1111/cpr.12875



26. Chakraborty P, Huang JTJ, Hiom K. DHX9 helicase promotes R-loop formation in cells with impaired RNA splicing. Nat Commun 2018; 9:4346
https://doi.org/10.1038/s41467-018-06677-1



27. Yuan W, Al-Hadid Q, Wang Z, et al. TDRD3 promotes DHX9 chromatin recruitment and R-loop resolution. Nucleic Acids Res 2021; 49:8573–91.
https://doi.org/10.1093/nar/gkab642



28. Abdelhaleem M, Maltais L, Wain H. The human DDX and DHX gene families of putative RNA helicases. Genomics 2003; 81:618–22.
https://doi.org/10.1016/s0888-7543(03)00049-1


29. Bourgeois CF, Mortreux F, Auboeuf D. The multiple functions of RNA helicases as drivers and regulators of gene expression. Nat Rev Mol Cell Biol 2016; 17:426–38.
https://doi.org/10.1038/nrm.2016.50


30. Putnam AA, Jankowsky E. DEAD-box helicases as integrators of RNA, nucleotide and protein binding. Biochim Biophys Acta Gene Regul Mech 2013; 1829:884–93.
https://doi.org/10.1016/j.bbagrm.2013.02.002



31. Fuller-Pace FV. DExD/H box RNA helicases: multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Res 2006; 34:4206–15.
https://doi.org/10.1093/nar/gkl460



32. Kang HJ, Eom HJ, Kim H, Myung K, Kwon HM, Choi JH. Thrap3 promotes R-loop resolution via interaction with methylated DDX5. Exp Mol Med 2021; 53:1602–11.
https://doi.org/10.1038/s12276-021-00689-6



33. Villarreal OD, Mersaoui SY, Yu Z, Masson JY, Richard S. Genome-wide R-loop analysis defines unique roles for DDX5, XRN2, and PRMT5 in DNA/RNA hybrid resolution. Life Sci Alliance 2020; 3:e202000762
https://doi.org/10.26508/lsa.202000762



34. Skourti-Stathaki K, Proudfoot NJ, Gromak N. Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol Cell 2011; 42:794–805.
https://doi.org/10.1016/j.molcel.2011.04.026



35. Zhao DY, Gish G, Braunschweig U, et al. SMN and symmetric arginine dimethylation of RNA polymerase II C-terminal domain control termination. Nature 2016; 529:48–53.
https://doi.org/10.1038/nature16469


36. Yu Z, Mersaoui SY, Guitton-Sert L, et al. DDX5 resolves R-loops at DNA double-strand breaks to promote DNA repair and avoid chromosomal deletions. NAR Cancer. 2020. 2:zcaa028
https://doi.org/10.1093/narcan/zcaa028



37. Sessa G, Gómez-González B, Silva S, et al. BRCA2 promotes DNA-RNA hybrid resolution by DDX5 helicase at DNA breaks to facilitate their repairdouble dagger. EMBO J 2021; 40:e106018
https://doi.org/10.15252/embj.2020106018



38. Leszczynska KB, Dzwigonska M, Estephan H, et al. Hypoxia-mediated regulation of DDX5 through decreased chromatin accessibility and post-translational targeting restricts R-loop accumulation. Mol Oncol 2023; 17:1173–91.
https://doi.org/10.1002/1878-0261.13431



39. Zhang C, Wang M, Li Y, Zhang Y. Profiling and functional characterization of maternal mRNA translation during mouse maternal-to-zygotic transition. Sci Adv. 2022. 8:eabj3967
https://doi.org/10.1126/sciadv.abj3967



40. Dang Y, Li S, Zhao P, et al. The lysine deacetylase activity of histone deacetylases 1 and 2 is required to safeguard zygotic genome activation in mice and cattle. Development. 2022. 149:dev200854
https://doi.org/10.1242/dev.200854


41. Ma P, Pan H, Montgomery RL, Olson EN, Schultz RM. Compensatory functions of histone deacetylase 1 (HDAC1) and HDAC2 regulate transcription and apoptosis during mouse oocyte development. Proc Natl Acad Sci USA 2012; 109:E481–9.
https://doi.org/10.1073/pnas.1118403109



42. Matsubara K, Lee AR, Kishigami S, et al. Dynamics and regulation of lysine-acetylation during one-cell stage mouse embryos. Biochem Biophys Res Commun 2013; 434:1–7.
https://doi.org/10.1016/j.bbrc.2013.03.083


43. Wang M, Chen Z, Zhang Y. CBP/p300 and HDAC activities regulate H3K27 acetylation dynamics and zygotic genome activation in mouse preimplantation embryos. EMBO J 2022; 41:e112012
https://doi.org/10.15252/embj.2022112012



44. Wu D, Dean J. Maternal factors regulating preimplantation development in mice. Curr Top Dev Biol 2020; 140:317–40.
https://doi.org/10.1016/bs.ctdb.2019.10.006



45. Aoki F. Zygotic gene activation in mice: profile and regulation. J Reprod Dev 2022; 68:79–84.
https://doi.org/10.1262/jrd.2021-129



46. Aoshima K, Inoue E, Sawa H, Okada Y. Paternal H3K4 methylation is required for minor zygotic gene activation and early mouse embryonic development. EMBO Rep 2015; 16:803–12.
https://doi.org/10.15252/embr.201439700



47. Lee H, You SY, Han DW, et al. Dynamic change of R-loop implicates in the regulation of zygotic genome activation in mouse. Int J Mol Sci 2022; 23:14345
https://doi.org/10.3390/ijms232214345



48. Suo L, Zhou YX, Jia LL, et al. Transcriptome profiling of human oocytes experiencing recurrent total fertilization failure. Sci Rep 2018; 8:17890
https://doi.org/10.1038/s41598-018-36275-6



- TOOLS






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





