 |
 |
| Anim Biosci > Volume 36(2); 2023 > Article |
|
Abstract
Objective
Methods
Results
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
The SRP Chair (PY) research programs are financially supported by grants from the Ministry of Agriculture Strategic Research Chair Program, the Natural Sciences and Engineering Research Council of Canada, SaskPulse Growers, the Prairie Oat Grower Association, the Saskatchewan Agricultural Development Fund, SaskCanola, SaskMilk, Saskatchewan Forage Network (SNK), Western Grain Research Foundation (WGRF) etc.
ACKNOWLEDGMENTS
Figure 1
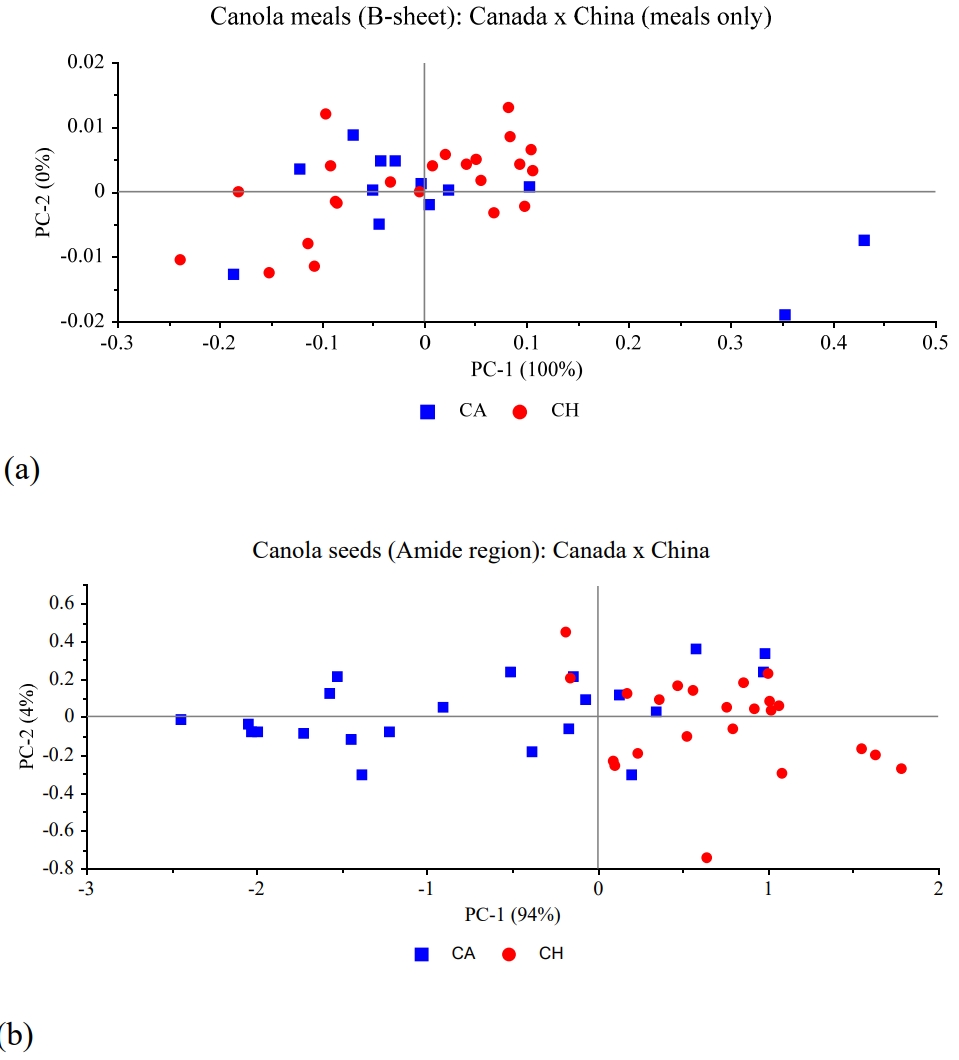
Figure 2
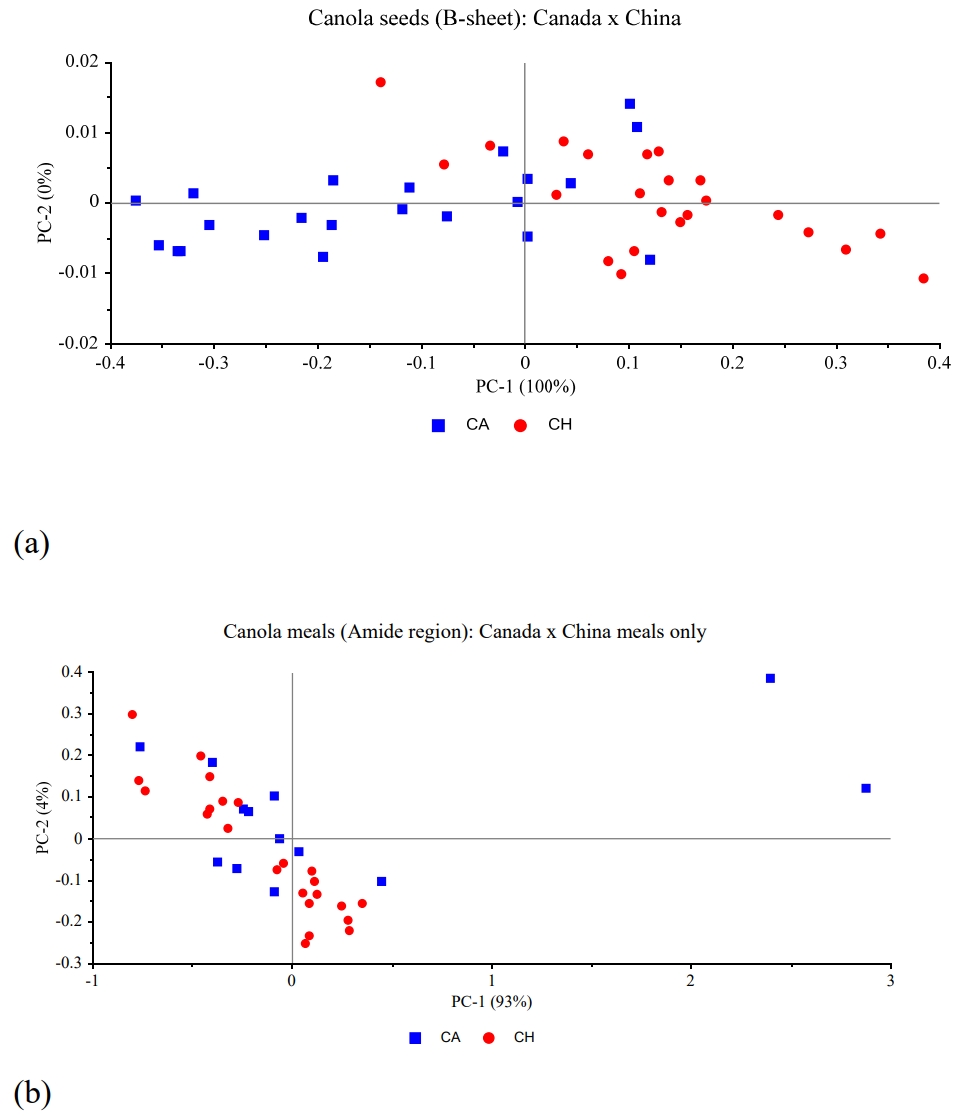
Table 1
|
Items Unit: AU |
Height | Area | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Total carbohydrate peaks (TC) | Cellulosic compounds peak (CEC) | Structural carbohydrates (ST) peaks | TC peak | CEC peak | STC peak | |||||||
|
|
|
|||||||||||
| Peak 1 (TC1) | Peak 2 (TC2) | Peak 3 (TC3) | Peak 4 (TC4) | Peak 1 (STC1) | Peak 2 (STC2) | Peak 3 (STC3) | Peak 4 (STC4) | |||||
| Canadian processing plants | ||||||||||||
| Plant 1 (M) | 0.48b | 0.46b | 0.35 | 0.16b | 0.06 | 0.06a | 0.06a | 0.11 | 0.06 | 76.18b | 3.80 | 18.24ab |
| Plant 2 (M) | 0.51ab | 0.49ab | 0.37 | 0.19ab | 0.06 | 0.03b | 0.05b | 0.11 | 0.12 | 81.56ab | 3.24 | 16.51b |
| Plant 3 (P) | 0.55a | 0.52a | 0.39 | 0.20a | 0.07 | 0.04ab | 0.06ab | 0.12 | 0.11 | 86.49a | 3.53 | 18.26ab |
| Plant 4 (P) | 0.51ab | 0.49ab | 0.38 | 0.19a | 0.07 | 0.04ab | 0.06a | 0.12 | 0.13 | 82.30ab | 3.51 | 18.58a |
| Plant 5 (P) | 0.51ab | 0.49ab | 0.37 | 0.18ab | 0.07 | 0.04ab | 0.06a | 0.11 | 0.13 | 80.94ab | 3.56 | 18.23ab |
| SEM | 0.011 | 0.010 | 0.010 | 0.010 | 0.003 | 0.005 | 0.002 | 0.005 | 0.019 | 2.010 | 0.141 | 0.460 |
| p-value | 0.010 | 0.041 | 0.047 | 0.028 | 0.253 | 0.041 | 0.013 | 0.204 | 0.071 | 0.022 | 0.096 | 0.032 |
| Meals vs pellets | ||||||||||||
| SEM | 0.061 | 0.058 | 0.054 | 0.050 | 0.015 | 0.027 | 0.011 | 0.023 | 0.097 | 10.521 | 0.739 | 2.310 |
| p-value | 0.027 | 0.041 | 0.016 | 0.023 | 0.038 | 0.324 | 0.052 | 0.028 | 0.047 | 0.023 | 0.974 | 0.022 |
| Chinese processing plants | ||||||||||||
| Plant A | 0.51 | 0.49 | 0.38 | 0.19b | 0.08 | 0.05 | 0.06 | 0.11 | 0.13 | 82.08 | 3.94 | 19.48 |
| Plant B | 0.52 | 0.49 | 0.38 | 0.19ab | 0.07 | 0.05 | 0.07 | 0.12 | 0.12 | 83.56 | 3.94 | 20.55 |
| Plant C | 0.52 | 0.49 | 0.38 | 0.20a | 0.07 | 0.04 | 0.06 | 0.12 | 0.12 | 84.48 | 3.82 | 19.13 |
| Plant D | 0.52 | 0.49 | 0.38 | 0.19ab | 0.07 | 0.05 | 0.07 | 0.12 | 0.12 | 83.61 | 3.86 | 19.98 |
| Plant E | 0.52 | 0.48 | 0.37 | 0.19ab | 0.07 | 0.05 | 0.07 | 0.12 | 0.12 | 82.79 | 3.77 | 19.88 |
| SEM | 0.006 | 0.007 | 0.004 | 0.003 | 0.002 | 0.003 | 0.003 | 0.002 | 0.003 | 0.828 | 0.150 | 0.472 |
| p-value | 0.377 | 0.603 | 0.250 | 0.040 | 0.258 | 0.194 | 0.165 | 0.101 | 0.281 | 0.288 | 0.834 | 0.250 |
| Overall | ||||||||||||
| CA Plants | 0.50 | 0.47 | 0.35 | 0.17 | 0.06 | 0.05 | 0.06 | 0.11 | 0.08 | 78.57 | 3.56 | 17.47 |
| CH Plants | 0.52 | 0.49 | 0.38 | 0.19 | 0.07 | 0.05 | 0.06 | 0.12 | 0.12 | 83.35 | 3.83 | 19.73 |
| SEM | 0.007 | 0.007 | 0.006 | 0.006 | 0.002 | 0.004 | 0.002 | 0.003 | 0.011 | 1.182 | 0.121 | 0.403 |
| p-value | 0.011 | 0.057 | 0.005 | 0.001 | <0.001 | 0.700 | <0.001 | 0.002 | 0.006 | 0.002 | 0.023 | <0.001 |
FTIR-ATR, Fourier transform infrared-attenuated total reflectance; TC, total carbohydrate; STC, structural carbohydrate; CEC, cellulosic compound; 1, 2, 3 and 4: correspond to the different peaks; SEM, standard error of the mean; CA, Canada; CH, China; M, meals; P, meals pelleted; Overall, compares only meals.
Table 2
|
Items Unit: AU |
Height | Area | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Total carbohydrate peaks (TC) | Cellulosic compounds peak (CEC) | Structural carbohydrates (ST) peaks | TC peak | CEC peak | STC peak | |||||||
|
|
|
|||||||||||
| Peak 1 (TC1) | Peak 2 (TC2) | Peak 3 (TC3) | Peak 4 (TC4) | Peak 1 (STC1) | Peak 2 (STC2) | Peak 3 (STC3) | Peak 4 (STC4) | |||||
| Canadian processing plants | ||||||||||||
| Plant 1 | 0.55 | 0.49 | 0.36 | 0.19 | 0.07 | 0.07b | 0.07 | 0.10b | 0.11 | 84.06 | 3.69 | 20.60 |
| Plant 2 | 0.55 | 0.53 | 0.37 | 0.18 | 0.07 | 0.09ab | 0.08 | 0.12ab | 0.03 | 87.67 | 4.36 | 22.77 |
| Plant 3 | 0.56 | 0.51 | 0.37 | 0.16 | 0.07 | 0.09ab | 0.08 | 0.12ab | 0.03 | 85.98 | 4.25 | 23.23 |
| Plant 4 | 0.55 | 0.51 | 0.37 | 0.16 | 0.07 | 0.10a | 0.09 | 0.12ab | 0.04 | 84.26 | 4.27 | 24.49 |
| Plant 5 | 0.53 | 0.51 | 0.37 | 0.16 | 0.07 | 0.10a | 0.09 | 0.13a | 0.02 | 83.20 | 4.35 | 24.94 |
| SEM | 0.244 | 0.214 | 0.019 | 0.018 | 0.005 | 0.008 | 0.005 | 0.007 | 0.033 | 3.906 | 0.246 | 1.281 |
| p-value | 0.915 | 0.655 | 0.981 | 0.664 | 0.954 | 0.009 | 0.050 | 0.044 | 0.189 | 0.873 | 0.225 | 0.075 |
| Chinese processing plants | ||||||||||||
| Plant A | 0.50 | 0.48 | 0.33 | 0.12 | 0.06 | 0.10 | 0.08 | 0.11 | 0.00 | 77.15 | 4.11 | 22.21 |
| Plant B | 0.51 | 0.48 | 0.34 | 0.14 | 0.06 | 0.10 | 0.09 | 0.12 | 0.00 | 78.63 | 4.10 | 22.86 |
| Plant C | 0.51 | 0.49 | 0.35 | 0.14 | 0.07 | 0.10 | 0.08 | 0.12 | 0.01 | 79.69 | 4.13 | 22.41 |
| Plant D | 0.49 | 0.48 | 0.33 | 0.13 | 0.07 | 0.10 | 0.08 | 0.12 | 0.00 | 76.94 | 4.29 | 22.44 |
| Plant E | 0.48 | 0.47 | 0.32 | 0.14 | 0.06 | 0.10 | 0.08 | 0.12 | 0.00 | 74.16 | 4.03 | 21.78 |
| SEM | 0.012 | 0.011 | 0.010 | 0.009 | 0.002 | 0.003 | 0.003 | 0.003 | 0.012 | 1.838 | 0.100 | 0.707 |
| p-value | 0.414 | 0.503 | 0.283 | 0.531 | 0.582 | 0.747 | 0.939 | 0.547 | 0.657 | 0.221 | 0.377 | 0.800 |
| Overall | ||||||||||||
| CA Plants | 0.55 | 0.51 | 0.37 | 0.17 | 0.07 | 0.09 | 0.08 | 0.12 | 0.05 | 84.81 | 4.16 | 23.08 |
| CH Plants | 0.50 | 0.48 | 0.34 | 0.14 | 0.06 | 0.10 | 0.08 | 0.12 | 0.00 | 77.59 | 4.14 | 22.40 |
| SEM | 0.010 | 0.010 | 0.009 | 0.007 | 0.001 | 0.003 | 0.002 | 0.003 | 0.011 | 1.737 | 0.083 | 0.592 |
| p-value | <0.001 | 0.005 | <0.001 | <0.001 | <0.004 | 0.033 | 0.044 | 0.100 | <0.001 | <0.001 | 0.804 | 0.284 |
Table 3
|
Items Unit: AU |
Height | Ratio | Height | Ratio | Area | Ratio | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||
| Amide I | Amide II | Amide I: Amide II | α-helix | β-sheet | α-helix: β-sheet | Amide | Amide I | Amide II | Amide I: Amide II | |
| Canadian processing plants | ||||||||||
| Plant 1 (M) | 0.35 | 0.18 | 1.99 | 0.32 | 0.32 | 0.99 | 50.76 | 27.02 | 9.04 | 3.08 |
| Plant 2 (M) | 0.38 | 0.21 | 1.88 | 0.35 | 0.34 | 1.00 | 53.36 | 28.46 | 10.25 | 2.93 |
| Plant 3 (P) | 0.39 | 0.22 | 1.93 | 0.37 | 0.36 | 1.00 | 55.50 | 29.42 | 10.45 | 3.01 |
| Plant 4 (P) | 0.40 | 0.22 | 1.93 | 0.38 | 0.37 | 1.02 | 57.19 | 30.56 | 10.90 | 2.93 |
| Plant 5 (P) | 0.39 | 0.23 | 1.78 | 0.37 | 0.35 | 1.03 | 54.64 | 29.24 | 10.94 | 2.79 |
| SEM | 0.025 | 0.030 | 0.140 | 0.031 | 0.027 | 0.022 | 2.320 | 2.797 | 1.769 | 0.205 |
| p-value | 0.193 | 0.283 | 0.481 | 0.124 | 0.132 | 0.538 | 0.236 | 0.209 | 0.422 | 0.664 |
| Meals vs pellets | ||||||||||
| SEM | 0.082 | 0.075 | 0.405 | 0.091 | 0.072 | 0.112 | 11.14 | 5.830 | 4.237 | 0.729 |
| p-value | 0.052 | 0.084 | 0.451 | 0.028 | 0.032 | 0.226 | 0.066 | 0.062 | 0.140 | 0.453 |
| Chinese processing plants | ||||||||||
| Plant A (M) | 0.40a | 0.22a | 1.85b | 0.37ab | 0.37a | 1.01b | 54.08ab | 29.79ab | 10.86a | 2.85b |
| Plant B (M) | 0.41a | 0.22a | 1.92ab | 0.38a | 0.37a | 1.02b | 56.74a | 31.19a | 10.98a | 2.99ab |
| Plant C (M) | 0.41a | 0.21a | 2.02a | 0.39a | 0.37a | 1.05ab | 55.84a | 30.97a | 10.96a | 2.98ab |
| Plant D (M) | 0.41a | 0.21a | 1.99a | 0.39a | 0.36a | 1.08a | 56.48a | 30.93a | 10.71a | 3.03a |
| Plant E (M) | 0.37b | 0.20b | 1.91ab | 0.35b | 0.34b | 1.05ab | 51.86b | 28.40b | 10.06b | 2.99a |
| SEM | 0.018 | 0.026 | 0.152 | 0.021 | 0.025 | 0.019 | 1.605 | 2.620 | 1.758 | 0.221 |
| p-value | 0.001 | <0.001 | 0.013 | <0.001 | <0.001 | 0.014 | 0.001 | 0.001 | 0.004 | 0.017 |
| Overall | ||||||||||
| CA Plants | 0.37 | 0.20 | 1.94 | 0.33 | 0.33 | 0.99 | 51.97 | 27.71 | 9.59 | 3.01 |
| CH Plants | 0.40 | 0.21 | 1.97 | 0.37 | 0.36 | 1.04 | 55.16 | 29.59 | 10.31 | 3.01 |
| SEM | 0.017 | 0.023 | 0.117 | 0.021 | 0.022 | 0.015 | 1.629 | 2.346 | 1.473 | 0.171 |
| p-value | 0.011 | 0.175 | 0.585 | 0.001 | 0.012 | 0.008 | 0.038 | 0.019 | 0.149 | 0.977 |
Table 4
|
Items Unit: AU |
Height | Ratio | Height | Ratio | Area | Ratio | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||
| Amide I | Amide II | Amide I: Amide II | α-helix | β-sheet | α-helix: β-sheet | Amide | Amide I | Amide II | Amide I: Amide II | |
| Canadian processing plants | ||||||||||
| Plant 1 | 0.33 | 0.16 | 2.19 | 0.33 | 0.30 | 1.10ab | 42.50 | 23.32 | 8.31 | 3.04 |
| Plant 2 | 0.32 | 0.16 | 2.02 | 0.31 | 0.28 | 1.12a | 41.66 | 22.74 | 8.92 | 2.72 |
| Plant 3 | 0.31 | 0.15 | 2.16 | 0.28 | 0.27 | 1.05bc | 41.11 | 21.72 | 7.80 | 3.10 |
| Plant 4 | 0.33 | 0.15 | 2.36 | 0.31 | 0.31 | 1.01c | 42.84 | 23.86 | 7.91 | 3.37 |
| Plant 5 | 0.34 | 0.15 | 2.37 | 0.32 | 0.30 | 1.06abc | 46.81 | 24.58 | 8.68 | 3.18 |
| SEM | 0.014 | 0.014 | 0.161 | 0.017 | 0.015 | 0.016 | 2.266 | 1.550 | 1.339 | 0.356 |
| p-value | 0.533 | 0.471 | 0.067 | 0.234 | 0.229 | <0.001 | 0.294 | 0.365 | 0.385 | 0.255 |
| Chinese processing plants | ||||||||||
| Plant A | 0.29 | 0.14 | 2.22 | 0.27 | 0.26 | 1.05 | 39.62 | 21.39 | 7.79 | 3.13 |
| Plant B | 0.31 | 0.14 | 2.42 | 0.29 | 0.28 | 1.06 | 43.21 | 22.98 | 7.98 | 3.42 |
| Plant C | 0.33 | 0.16 | 2.24 | 0.32 | 0.30 | 1.08 | 45.52 | 24.88 | 8.85 | 3.20 |
| Plant D | 0.33 | 0.16 | 2.14 | 0.31 | 0.29 | 1.08 | 43.28 | 23.82 | 9.18 | 2.81 |
| Plant E | 0.35 | 0.16 | 2.35 | 0.33 | 0.31 | 1.07 | 48.08 | 25.91 | 9.04 | 3.17 |
| SEM | 0.017 | 0.017 | 0.212 | 0.021 | 0.018 | 0.024 | 3.392 | 1.983 | 1.598 | 0.456 |
| p-value | 0.135 | 0.100 | 0.414 | 0.136 | 0.083 | 0.736 | 0.248 | 0.205 | 0.152 | 0.607 |
| Overall | ||||||||||
| CA Plants | 0.33 | 0.15 | 2.23 | 0.31 | 0.29 | 1.07 | 42.68 | 23.23 | 8.22 | 3.14 |
| CH Plants | 0.33 | 0.15 | 2.28 | 0.31 | 0.29 | 1.07 | 44.79 | 23.87 | 8.38 | 3.17 |
| SEM | 0.009 | 0.013 | 0.151 | 0.014 | 0.014 | 0.010 | 1.752 | 1.358 | 1.311 | 0.317 |
| p-value | 0.894 | 0.532 | 0.471 | 0.962 | 0.688 | 0.988 | 0.186 | 0.433 | 0.610 | 0.839 |






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





